A.P. Inkov, Ph.D. tech. Sciences, ECOTERM LLC
Ventilation systems, heating and air conditioning (VOK) should provide optimal microclimate conditions and air environment premises of a hospital, maternity hospital or other hospital. When designing, building (reconstructing) and operating FOC systems, one should use the main provisions of the existing special normative documents, as well as a number of other documents approved by the Russian Ministry of Health. At the same time, the EQA systems for medical institutions (HCI) in accordance with Russian norms have a number of features compared to other public buildings and structures. Some of them are listed below.
1. The use of vertical collectors for both supply and exhaust systems is not allowed in the buildings of healthcare facilities.
2. Removal of air from operating rooms, anesthesia, resuscitation, birth and x-ray rooms is carried out from two zones (upper and lower).
3. Relative humidity and temperature of operating units is maintained constantly and around the clock.
4. In hospital wards, relative air humidity is standardized only for the winter period.
5. Air recirculation is not allowed in the buildings of healthcare facilities in VOK systems.
6. The temperature of the heat carrier for water heating systems must correspond to the purpose of the building.
7. Level sound pressure from ventilation systems in wards and operating rooms of hospitals should not exceed 35 dBA.
In view of the foregoing, it is clear that only specialized design organizations with a library of regulatory documents and some practical experience can carry out a high-quality design of the FQA system.
Below we take a closer look at the most complex issue with design , postoperative wards, resuscitation rooms, intensive care wards, delivery boxes, anesthetic and other rooms, classified according to the standards to the cleanliness category "OC". In these rooms, ventilation and air conditioning are mandatory, and at the same time, the frequency of air exchange is determined by calculation from the conditions of heat release assimilation, but not less than ten times the exchange
(see Table 1 for norms).
Table number 1. Estimated temperatures, air exchange rates, cleanliness categories in medical institutions
It should be immediately noted that the classification of premises according to the degree of air purity adopted in the work is outdated and requires processing in accordance with the current regulations.
new standard adopted and introduced in Russia on May 18, 2000 and harmonized with the international standard ISO 14644-1-99. This article will use the terms and definitions of this standard, in which purity classes are limited to ISO class 1 ( upper class) up to ISO class 9 (lowest class).
It is known that prolonged stay of patients in conventional surgical and therapeutic hospitals is dangerous for them. After some time in the hospital, they become carriers of the so-called hospital strains and carriers of pathogens of various infections. This also applies to staff. medical institutions. Such methods of prevention and treatment of infections, such as antibiotics, immune and hormonal preparations, wet cleaning of premises with antiseptic solutions, ultraviolet irradiation, etc., do not give the desired effect.
A clean room compared to these methods has a fundamental difference. It is not aimed at fighting and destroying already existing microorganisms in the room. It does not allow them there, and microorganisms emanating from patients or medical personnel are immediately removed from the room by air flow. The goal of clean operating rooms is to reduce the growth of microbial contamination, primarily in the area of the operating room and instrument tables.
According to the modern classification, operating rooms can be classified as clean rooms (CP) of ISO class 5 and above. The class of a cleanroom is characterized by a classification number that determines the maximum allowable countable concentration of aerosol particles of a certain size in one cubic meter air. A particle is understood as a solid, liquid or multi-phase object with a size of 0.05 to 100 microns. When classifying CP, non-living particles with a size of 0.1 to 5 microns are considered. A cleanroom may contain one or more clean zones (a clean zone may be open or enclosed) and be located both inside and outside the cleanroom.
According to the standard, a cleanroom is a room in which the concentration of airborne particles is controlled and which is constructed and used in such a way as to minimize the intake, emission and retention of particles inside the room, and in which other parameters are controlled, as necessary, such as temperature, humidity and pressure.
In accordance with the standard, three time phases of the creation and existence of a cleanroom should be distinguished:
1. As-built: state in which the cleanroom system is complete, all service systems are connected, but not present production equipment, materials and personnel.
2. Equipped (at-rest): the state in which the cleanroom system is equipped and debugged in accordance with the agreement between the customer and the contractor, but there is no staff.
3. Operational: the state in which the cleanroom system is operating in the intended manner, with a specified number of personnel working in accordance with the documentation.
This above division is of fundamental importance in the design, construction, certification and operation of clean rooms. Particulate air purity in a cleanroom or clean area should be determined from one (or more) of the three cleanroom conditions. When designing and building medical institutions, we will be most interested in the latter, operational condition State of emergency.
The air around us contains a large number of both living and non-living particles, differing in nature and size. In the standard, when determining the air purity class in a clean room, the concentration of non-living aerosol particles ranging in size from 0.1 to 5.0 microns is taken into account. When assessing the class of air purity in operating rooms important criterion is the number of living microorganisms in it, so this issue needs to be considered in more detail.
The paper analyzes the main sources of air micropollution. Foreign statistical data are given, showing that there is approximately one microorganism per 1,000 suspended aerosol particles. It is said that in view of the multiplicity of factors affecting microbial contamination, these data are of an approximate, probabilistic nature. But nevertheless they give an idea of the relationship between the number of non-living particles and the number of microorganisms in the air.
Airborne particle purity classes for cleanrooms and clean areas
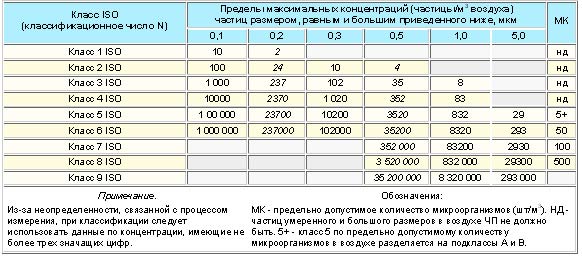
To assess the required class of air purity in operating rooms, depending on the volume concentration of microorganisms in it, you can use the data of the summary table. 2 standards.
Clean rooms class 5 in table. 2 are divided into two subclasses:
- Subclass A - with the maximum allowable number of microorganisms not more than 1 (achieved in a unidirectional air flow).
- Subclass B - with the maximum allowable number of microorganisms not more than 5.
Higher class cleanrooms (classes 4 to 1) should be free of microorganisms at all.
In order to move on to the consideration of practical issues that are of most interest to designers of HVAC systems, we will once again consider some of the requirements imposed by regulatory documents on ventilation and air conditioning systems in emergency situations. In passing, we note that in addition to the requirements for VC systems, designers must also know and fulfill the entire list of other mandatory requirements for emergency situations: requirements for planning solutions, requirements for the design and materials of emergency situations, requirements for equipment for emergency situations, requirements for engineering systems, requirements for medical personnel and technological clothing, etc. Due to the limited scope of this article, these issues are not considered here.
Below is a list of only some of the basic requirements for ventilation and air conditioning systems of the emergency.
1. The air supply system in the emergency room from 1 to 6 classes, as a rule, should provide the organization of air exchange with a vertical unidirectional flow. For class 6, non-unidirectional airflow is possible. The standard provides a definition: unidirectional air flow - air flow with, as a rule, parallel jets (streamlines) passing in the same direction with the same speed in cross section. The terms "laminar" and "turbulent" flow are not recommended for characterizing air flows in the CP.
2. Coverings of air ducts and their structures located in clean rooms, as well as coverings of filter chambers and their structures must allow periodic treatment with disinfectant solutions. This requirement is mandatory for controlled microbial contamination emergencies.
3.
must have automatic regulation temperature and humidity, blocking, remote control, signaling.
4. In a CV with unidirectional vertical flow, the number of openings that discharge air flows from the CV is selected in accordance with the need to ensure the verticality of the air flows.
In addition to the above requirements for ventilation and air conditioning systems operating rooms should also be added:
- The requirement to use multi-stage filtration of the air supplied from outside (at least 3 stages) and use as final filters high efficiency class not less than H12 .
- The requirement to provide the necessary speed of a unidirectional flow of 0.2-0.45 m/s at the outlet of .
- The requirement for a positive differential pressure in the operating room and surrounding areas in the range of 5-20 Pa.
New construction and renovation of hospital operating theaters to meet all the requirements of class 5 and higher cleanrooms is very costly. The cost of only the enclosing structures of one operating room with a "laminar" flow is from several tens of thousands of US dollars and more, plus the cost of a central air conditioner system. If abroad, standards for air purity have been developed and are in force in various premises hospitals (in Germany and Holland, the number of operating clean operating rooms taken together is more than 800), then in our country the issue of setting requirements for equipping the operating room with all systems is often decided at the level of the head physician of the hospital and his deputies, who are sometimes simply unfamiliar with the regulatory requirements for clean rooms , and their choice is determined primarily by financial capabilities, especially in budgetary organizations.
Having considered the complex general requirements to the ventilation and air conditioning systems of the state of emergency, it can be concluded that proper organization air flow (unidirectional, non-unidirectional) is one of the most important conditions for ensuring the required air purity and patient safety. The air flow must carry away all particles emitted by people, equipment and materials from the clean area.

On fig. 1 shows the most common air supply schemes in the operating room and their comparative analysis in terms of bacterial contamination is performed. Scheme 1d provides unidirectional vertical airflow, the other schemes - non-unidirectional airflow.
The quality of the unidirectional air flow is greatly influenced by the design of the distributor through which the air flows directly into the clean room. This distributor is located directly between the HEPA filters and the frequency converter. It can be made in the form of a lattice or in the form of a single or double mesh of metal or synthetic material. Importance has the size of the hole and the distance between the holes through which air passes. The greater this distance, the worse quality flow (Fig. 2).

If in rooms with unidirectional air flow the air diffuser occupies the entire area of the ceiling above the operating area, then in rooms of a lower cleanliness class with non-unidirectional air flow, supply diffusers occupy only a part of the ceiling, sometimes quite a small one. Exhaust grilles can also be located in various ways (schemes 1a, 1b, 1c, 1e). In this case, only the methods of numerical mathematical modeling make it possible to take into account all the variety of influencing factors on the picture of air flows and evaluate how the position of filters, equipment, heat sources (lamps, etc.) affects air flows and the class of cleanliness in various areas of the operating room.
Different kinds versions of ceiling diffusers with a filter for clean rooms manufactured by GEA are shown in fig. 3.

These diffusers are equipped with hermetic valves to isolate the air filter from the rest of the air conditioning system. This allows you to replace the air filter without turning off the air conditioner. The tightness of the air filter installation in the diffuser cell can be monitored using a tightness sensor. Sensors are also built in to measure the differential pressure across the filter.
Main results comparative analysis various ways filing clean air in the operating rooms according to the work are presented in fig. four.

The figure shows the measurement results for different flows and two limit curves that must not be exceeded for operating rooms type A (especially high requirements according to DIN 1946, part 4, edition 1998) or type B (high requirements).
Using the indicator of microbial contamination with a known volumetric air flow, it is possible to calculate microbial contamination (CFU/m3)*: K=n.Q.ms/V,
where:
K - colony-forming units per 1 m 3 of air;
Q is the initial intensity of microbial sources;
ms - indicator of microbial contamination;
V - volumetric air flow;
n is the number of personnel in the operating room.
The following conclusions are made in the work. Separate diffusers or perforated ceilings supply clean air and mix it with polluted air (dilute method). Indicators of microbial contamination are at best around 0.5. With a unidirectional “laminar” air flow, a microbial contamination index of 0.1 or less is achieved.
As mentioned above, with radial outlet diffusers on the ceiling in the room, a mixed flow is created. This output at a volume flow rate of 2,400 m3/h meets the class B standard requirements, and a flow rate of 2,400 m3/h can be taken as the minimum allowable clean air flow rate supplied to the operating area (this flow rate is taken as the reference volume flow rate in the standard DIN 4799, designed to evaluate and compare different types of ceilings).
To date, ceiling-type mesh air distribution devices for creating a unidirectional air flow for operating rooms are produced by a number of companies, for example, , ADMECO AG, ROX LUFTTECHIK GmbH, etc.

On fig. 5 shows a typical structural diagram of such an air distribution device (laminar ceiling).
In practice, the most common size of such devices (ceilings) is from 1.8x2.4 m 2 to 3.2x3.2 m 2, and the latter size is the most common abroad. For example, for1.8x2.4 m 2 required flow air will be 3100 m 3 / h (at a speed of air outlet from the device of 0.2 m / s). From the practice of designing several operating theaters at the Moscow Central Institute of Traumatology and Orthopedics (CITO) by our design department, we can conclude that such a flow rate corresponds to a 25-fold air exchange in a room with an area of 30-40 m 2 and always exceeds the calculated flow rate necessary for the assimilation of heat excesses characteristic for typical recruitment and equipment for these premises.
Our data are in good agreement with the data of the work, which shows the amount of heat release of 1.5-2.0 kW, typical for operating rooms, as well as the calculated value of clean air supply of 2000-2500 m 3 / h (17-20 times per hour). In this case, the temperature of the supply air should differ from the temperature of the operating area by no more than 5 degrees.
The larger the size laminar ceiling in the above range, the higher the degree of patient safety, however, at the same time, capital and operating costs increase significantly. Abroad, a reasonable compromise is widely used - the introduction of an air recirculation system in the operating room through highly efficient HEPA filters built into the "laminar" ceiling. This allows you to increase the size of the "laminar" ceiling up to 3.2x3.2 m 2 while maintaining low capital and operating costs for the central air conditioner.
For example, operating rooms are being designed, where, when outdoor air is supplied with an air conditioner of 1200-2000 m 3 /h, the circulation flow in the operating room is up to 8000 m 3 /h, while energy supply costs are significantly reduced. Enlargementup to 3.2x3.2 m 2 allows you to include in the sterile area not only the patient, but also the instrument table and the working staff, especially if you also use special enclosing plastic aprons (Fig. 6).

Another advantage of the system for using air circulation in the operating room (which is allowed in accordance with part 4 of DIN 1946) is the possibility at night, when the operating room equipment is not in use, to turn off the air conditioner to the outside air completely or partially, using only the equipment (fan ) internal system circulation of clean air, while consuming approximately 400 watts of power.
Speaking about energy saving in EQA systems for operating rooms in hospitals, it should be noted the work of prof. O. Ya. Kokorina. In this work, it is also proposed to use a circulating mixing and cleaning supply unit, but this scheme was analyzed only for the variant of supplying a non-uniform flow of clean air in the operating room according to the scheme shown in Fig. 1a.
Given the energy attractiveness of the proposed scheme, designers may encounter problems during its implementation with the need to place a mixing and cleaning unit with a capacity of 2,400 m3/h in rooms near the operating room, as well as problems with the distribution of supply and exhaust systems, since a monoblock supply and exhaust unit is used.
* The term CFU means "colony forming units" (CFU - Colony Forming Units) and is more accurate characterization microbial contamination. Clean room technology allows to ensure the level of microbial contamination of less than 10 CFU/m 3 . There is evidence that reducing microbial air pollution in the area of the operating table by 10 times reduces the risk of infection by 2%.
Example:
Q=30,000 microbes per person per hour (assumption). For 8 people in the operating room with µs=0.1 and a volume flow of 2400 m 3 /h K=8x30000x0.1/2400=10 CFU/m3.
Published in ABOK magazine
Very often, the term "clean rooms" is applied to operating units.
In all "clean rooms" it is necessary to strictly comply with certain requirements for the frequency of air exchange, air humidity and cleanliness. In such rooms, the values of humidity and air temperature are very accurately observed. In operating units of a general surgical profile, which include the birth, anesthesia and operating rooms, temperature regime within 20 - 23 degrees Celsius, and the relative humidity should be 55 - 60%. These rules are followed for several important reasons. At relative air humidity below 55%, the process of formation of static electricity begins in these rooms. In parallel with this, during the medical and technological course of operations, gases are formed that are used for anesthesia. When a critical level of static electricity is reached, these gases can explode. Also, at low relative humidity, unsatisfactory well-being of medical personnel is possible. Therefore, to prevent this, it is necessary to maintain a constant temperature in the room. To create the most comfortable thermal conditions for doctors working in overalls (bandages, suits, gowns, gloves), which impair heat transfer, the temperature should not exceed 23 degrees.
According to a number of microbiological studies, it was found that as a result of the release of moisture by a person, the indicator of the intensity of the formation of bacteria in the human body increases significantly. According to established standards, air mobility in the area of the patient's head should not exceed 0.1 - 0.15 m / s. Due to the fact that postoperative wound infections are still quite common, all anti-epidemiological requirements with the use of antibiotics are observed in operating rooms, and strict requirements are imposed on climatic installations.
Now there is a tendency to locate "clean rooms" away from the facades, in the central part of the building, where there are no heat exchange processes through the fence with the external environment. In order to compensate for excess heat in such rooms, it is necessary to supply fresh air volume up to 2500 cubic meters / h (up to 20 times per hour with standard sizes operating room). An important fact is that the supply air temperature can only exceed the room temperature by 5 degrees. According to microbiological studies, this amount of fresh air will be enough to dilute and remove the bacterial flora.
Since the air supplied to operating rooms must be absolutely sterile, its purification is of particular importance. Filters are a very important component of the climate system in the premises of "clean rooms". It is with their help that the desired degree of air purity is achieved in the room. Thanks to filters with different degrees of purification (coarse, fine at the first and second stages), the air undergoes a three-stage purification. At the stage of the third stage, thanks to the use of microfilters and filters, the incoming air reaches required level fine cleaning. To extend the service life of the main filters, filters with a lower degree of purification are installed, made in the form of a preliminary cycle.
The widest range of quality air purifiers designed and manufactured in Russia, which are so indispensable for creating the necessary conditions in operating rooms, is presented in
The question of a special approach to the organization of air conditioning and ventilation systems of "clean" rooms is due to the very essence of this term.
"Clean" rooms are called laboratories in food, pharmaceutical and cosmetic industries, in research institutes, experimental rooms, in enterprises for the development and production of microelectronics, etc.
In addition, “clean” rooms include rooms in medical and preventive treatment facilities (MPIs): operating rooms, maternity, resuscitation, anesthesia rooms, X-ray rooms.
Requirements for a "clean room" and cleanliness class
On the this moment developed and operates GOST R ISO 14644-1-2000, which is based on international standard ISO 14644-1-99 Cleanrooms and associated controlled environments. In accordance with this document, all companies and organizations responsible for the ventilation and air conditioning of such premises should work.
The standard describes the requirements for a "clean room" and cleanliness class - from ISO 1 (highest class) to ISO 9 (lowest class). The purity class is determined depending on the permissible concentration of suspended particles in the air and their size. So, for example, the cleanliness class of operating rooms is from 5 and above. To determine the cleanliness class, the number of microorganisms in the air is also counted. For example, in class 1 rooms, there should be no microorganisms at all.
A “clean” room should be arranged and equipped in such a way as to minimize the entry of suspended particles into the room, and in case of entry, isolate them inside and limit the exit to the outside. In addition, these rooms must constantly and continuously maintain the desired temperature, humidity and pressure.
Features of ventilation and air conditioning for "clean" rooms
Based on the foregoing, the following features of ventilation and air conditioning systems are distinguished:
- In "clean" and medical rooms, it is prohibited to install air conditioners with air recirculation, only the supply type. The installation of split systems is allowed in the administrative premises of healthcare facilities and laboratories.
- Precision air conditioners are often used to provide and maintain accurate temperature and humidity parameters.
- The design and material of air ducts, filter chambers and their elements must be adapted for regular cleaning and disinfection.
- A multi-stage filtration system (at least two filters) must be installed in the air conditioning and ventilation network and high-efficiency HEPA final filters (High Efficiency Particular Airfilters) should be used.
Air filters differ depending on the stages of cleaning: 1 stage (coarse cleaning) 4-5; 2 stages (fine cleaning) from F7 and above; 3 stages - high efficiency filters above H11. Accordingly, the first stage filters take on outdoor air- they are installed at the air inlet to the supply unit and provide protection of the supply chamber from particles. The second stage filters are installed at the outlet of the supply chamber and protect the air duct from particles. Third stage filters are installed in the immediate vicinity of the serviced premises.
- Ensuring air exchange - the creation of excess pressure in relation to neighboring rooms.
The main tasks of the ventilation and air conditioning system for clean rooms are: removal of exhaust air from the premises; provision of supply air, its distribution and volume control; preparation of supply air according to the specified parameters - humidity, temperature, cleaning; organization of the direction of air movement based on the characteristics of the premises.
In addition to the air preparation and distribution system, the design of a "clean" room involves a whole complex additional elements: enclosing structures - hygienic wall enclosures, doors, hermetic ceilings, antistatic floors; control and dispatching system for supply and exhaust systems; a number of other special engineering equipment.
The design and installation of air preparation and distribution systems should be carried out only by specialized companies that have experience in such work, comply with all GOSTs and requirements, and ensure A complex approach to the organization of "clean" rooms. One contractor should ideally perform the work of design and construction, assembly and installation, commissioning and training of personnel in the specifics of being on the premises.
How to choose a contractor
To select a contractor, you need:
- find out if the company has experience in implementing GMP (Good Manufacturing Practice) standards or ISO 9000 standards;
- get acquainted with the experience of the company and with the portfolio of projects for the organization of "clean" rooms, which it carried out;
- request available distribution certificates, certificates of conformity to GOSTs, SRO approvals for design and installation work, licenses, technical regulations, cleanliness protocols and work permits;
- get to know the team of specialists who are engaged in design and installation;
- find out the conditions of warranty and post-warranty service.
Is it possible to use glycol in supply ventilation systems?
When designing buildings in areas with an estimated outdoor air temperature of -40 ° C and below (according to parameters B), it is allowed to use water with additives that prevent it from freezing. Accordingly, the application aqueous solution glycol is possible to eliminate the risk of freezing of air heaters.
Are there regulations for MRI rooms?
There are no special rules.
Are there rooms in medical buildings with category A for explosion and fire hazard?
The classification of premises of health care facilities by production categories according to ONTP 24-86 is given in PPBO 07-91 "Rules fire safety for healthcare institutions. In accordance with them, category A includes: premises for the storage of flammable liquids, storage gas cylinders, paint shops, accumulator (charging).
What heating devices are used in the wards of psychiatric hospitals?
Appliances should be used with a smooth surface that is resistant to daily exposure to detergents and disinfectants, excluding the accumulation of dust and microorganisms in all chambers.
How to maintain humidity in the premises when using ventilation systems?
For ward rooms during the cold season, for example, steam humidifiers can be used.
Is it possible to use split systems and fan coils in the premises of medical institutions?
With regard to split systems: “The use of split systems is allowed if there are high-efficiency filters (H11-H14) with mandatory observance of the rules of routine maintenance. Split systems must have a positive sanitary and epidemiological conclusion issued in accordance with the established procedure, that is, a certificate for the possibility of use in medical institutions. We can recommend the installation of split systems and fan coil units in administrative and auxiliary premises. Application this equipment in rooms for medical purposes, it does not allow to provide the required air mobility (0.15–0.2 m/s), in addition, fan coil units create a noise background that exceeds the permissible values (There are known cases of fan coil units to remove excess heat from equipment in the technical rooms of the KRT.)
Is there a clear requirement for a mandatory certificate for equipment for ventilation and air conditioning systems used in medical institutions?
There are no such requirements in the existing regulatory literature, however, medical equipment should be accepted for installation in a medical facility.
How to design ventilation in small built-in or attached dental departments occupying a floor or part of a floor in a building?
An independent supply and exhaust ventilation system for the dental department should be provided, the flow into the X-ray room is allowed from common system supply ventilation with the installation of a check valve, the hood should be provided independently. In operating rooms, an independent air conditioning system is required with three stages of supply air purification and the use of an H class filter at the final stage.
Is it possible to serve with one supply system the premises of operating rooms that are part of different departments (“dirty”) located on different floors?
As a rule, these are departments of various technological purposes. The cleanliness class A must be ensured in the operating room. To avoid the transfer of infection of one kind or another between operating rooms through the ventilation system, each operating room (operating unit of each department) should be serviced independently for the case under consideration. supply and exhaust system. If there are several operating rooms in one operating unit, they should be combined to serve one ventilation system.
Is it necessary to comply with the requirements for operating theaters in polyclinics the same as the requirements for operating theaters in hospitals?
Yes, it should. The operating room of the polyclinic is considered as a small operating room, in which air should be supplied through air distributors of a slightly turbulent flow.
What filters are used in health care facilities?
To ensure the required class of room cleanliness, it is necessary to provide for the installation of filters and air disinfection devices in ventilation and air conditioning systems.
Ventilation and air conditioning systems of class A and B rooms should be equipped with a three-stage system for cleaning and disinfecting supply air; rooms of other classes may be equipped with a two-stage system.
Air purification filters are used for individual filtration stages. Air filters general purpose(coarse and fine filters), as a rule, are used depending on the purification stage:
For stage 1 - a coarse cleaning group of a class not lower than G4 pocket type or F5 (or higher, as an option) depending on the pollution of the outside air;
For stage 2 - fine cleaning groups of class not lower than F7;
For stage 3 - a high efficiency group of a class not lower than H11 and / or air disinfection devices with an efficiency of inactivation of microorganisms and viruses of at least 95%.
When used as a 1st stage filter of class F5 and above, it is recommended (to prolong the service life of filters of the 2nd stage) that an additional pre-filter of class G3 or G4 be installed before the 1st stage filter.
Filters of purification stages 1 and 2 are placed directly in the supply ventilation or air conditioning systems:
Stage 1 - at the outside air inlet to the supply unit to protect the elements of the supply chamber from particles;
Stage 2 - at the exit from air handling unit to protect air ducts from particles.
Filters of purification stage 3 are placed as close as possible to the serviced premises or in the serviced premises itself after the air disinfection device (if necessary).
When choosing an air purification scheme for rooms of cleanliness classes A and B, it is necessary to take into account the indicators of background dust concentrations in atmospheric air requested from the territorial bodies of Roshydromet. The choice of an air purification scheme is carried out in agreement with the territorial bodies of Rospotrebnadzor.
How to produce air humidification?
In accordance with the above standards, air humidification should be carried out with steam (steam generator). Humidification of the air with water is permissible provided that it is disinfected.
The design of air humidifiers and their location should exclude the formation of condensate and drops of moisture after the humidifier and their entry into the supply ventilation system. Air humidifiers of nozzle or film type are installed before the final filtration stage. In the case of air humidification with steam, it is recommended to install the steam distribution device directly in the air duct. These devices should be placed in a place accessible for maintenance, cleaning and disinfection.
The make-up steam humidifier is connected to the water supply. To ensure reliable operation, it must comply with the manufacturer's water quality requirements.
To reduce the concentration of microorganisms, disinfection of water should be carried out.
What air conditioners should be installed in health facilities?
The equipment of air conditioning (ventilation) systems must be of medical design.
What is going on with us, no one knows. The picture in our hospitals is certainly much worse. Judging by the level of current industry regulations, our healthcare has not yet come to an understanding of the problem. And the problem is clear. It was put in the magazine "Technology of Purity", No. 1/96, 10 years ago. In 1998, ASINCOM developed the Standards for Air Cleanliness in Hospitals based on foreign experience.
In the same year they were sent to the Central Research Institute of Epidemiology. In 2002, this document was submitted to the State Sanitary and Epidemiological Supervision. There was no response in both cases. But in 2003, SanPiN 2.1.3.1375-03 “Hygienic requirements for the placement, arrangement, equipment and operation of hospitals, maternity hospitals and other medical hospitals” was approved - a backward document, the requirements of which sometimes contradict the laws of physics (see below).
The main objection to the introduction of Western standards is "there is no money." It is not true. There is money. But they don't go where they need to go. A decade of experience in certifying hospital premises by the Clean Rooms Certification Center and the Clean Room Testing Laboratory has shown that the actual cost of operating rooms and intensive care units exceeds, sometimes several times, the costs of facilities built according to European standards and equipped with Western equipment. At the same time, the objects do not correspond to the modern level. One of the reasons is the lack of a proper regulatory framework.
Existing standards and norms
Clean room technology has been used in Western hospitals for a long time. As early as 1961 in the UK, Professor Sir John Charnley equipped the first "greenhouse" operating room with an air flow velocity of 0.3 m/s descending from the ceiling. This was a radical means of reducing the risk of infection in patients undergoing hip transplantation.
Prior to this, 9% of patients had infection during surgery, and repeated transplantation was required. It was a true tragedy for the sick. In the 70-80s. cleanliness technology based on ventilation and air conditioning systems and the use of high-efficiency filters has become an integral element in hospitals in Europe and America. At the same time, the first standards for air purity in hospitals appeared in Germany, France and Switzerland. The second generation of standards based on modern level knowledge.
Switzerland
In 1987, the Swiss Institute for Health and Medical Institutions (SKI - Schweizerisches Institut fur Gesundheits und Krankenhauswesen) adopted the "Guidelines for the construction, operation and maintenance of air preparation systems in hospitals" - SKI, Band 35, "Richtlinien fur Bau, Betrieb und Uberwachung von raumlufttechnischen Anlagen in Spitalern. The management distinguishes three groups of premises - tab. one.
In 2003, the Swiss Society of Heating and Air Conditioning Engineers adopted the guideline SWKI 99-3 "Heating, ventilation and air conditioning systems in hospitals (design, construction and operation)". Its essential difference is refusal to ration air purity by microbial pollution (CFU) to evaluate the operation of the ventilation and air conditioning system. The evaluation criterion is the concentration of particles in the air (not microorganisms).
The manual establishes clear requirements for the preparation of air for operating rooms and provides an original method for assessing the effectiveness of cleanliness measures using an aerosol generator. Detailed Analysis guidelines are given in the article by A. Brunner in the journal "Technology of Purity", No. 1/2006.
Germany
In 1989, Germany adopted the DIN 1946 part 4, “Cleanroom technology. Clean air systems in hospitals” – DIN 1946, Teil 4. Raumlufttechik. Raumlufttechishe Anlagen in Krankenhausern, Dezember, 1989 (revised 1999). A draft DIN standard has now been prepared containing purity values for both micro-organisms (sedimentation method) and particles.
The standard regulates in detail the requirements for hygiene and cleanliness methods. Classes of premises Ia (highly aseptic operating rooms), Ib (other operating rooms) and II have been established. For classes Ia and Ib, the requirements for the maximum allowable air pollution by microorganisms (sedimentation method) are given - see table. 2. The requirements for filters for various stages of air purification are established: F5 (F7) + F9 + H13.
The Society of German Engineers VDI has prepared a draft standard VDI 2167, part "Equipment of hospital buildings - heating, ventilation and air conditioning". The draft is identical to the Swiss manual SWKI 99-3 and contains only editorial changes due to some differences between "Swiss" German and "German" German.
France
The air purity standard AFNOR NFX 90-351, 1987 in hospitals was adopted in France in 1987 and revised in 2003. The standard sets limits for the concentration of particles and microorganisms in the air. The particle concentration is determined by two sizes: ≥ 0.5 µm and ≥ 5.0 µm. Cleanliness is an important factor only in the equipped state of cleanrooms.
For more details on the requirements of the French standard, see the article by Fabrice Dorchies "France: the standard for clean air in hospitals" (Journal "Cleanliness Technology", No. 1/2006). The listed standards detail the requirements for operating rooms, set the number of filtration stages, filter types, laminar zone sizes, etc.
Hospital cleanroom design is based on the ISO 14644 series of standards (previously based on Fed. Std. 209D).
Russia
In 2003, SanPiN 2.1.3.1375-03 "Hygienic requirements for the location, arrangement, equipment and operation of hospitals, maternity hospitals and other medical hospitals" was adopted. Some of the requirements of this document are puzzling. For example, Appendix 7 establishes sanitary and microbiological indicators for rooms of different cleanliness classes - see table. 5.
In Russia cleanroom cleanliness classes were established by GOST R 50766-95, then GOST R ISO 14644-1-2001. Part 1. Classification of air purity. It is logical to expect that industry documents must comply with the national standard, not to mention the fact that the definitions of “conditionally clean”, “conditionally dirty” for cleanliness classes, “dirty ceiling” for ceilings look strange.
SanPiN 2.1.3.1375-03 establishes for "especially clean" rooms (operating rooms, aseptic boxes for hematological, burn patients) the indicator of the total number of microorganisms in the air, CFU / m 3, before starting work (equipped state) "no more than 200". And the French standard NFX 90-351 is no more than 5. These patients should be under unidirectional (laminar) air flow.
In the presence of 200 CFU/m 3 the patient in a state of immunodeficiency (aseptic box of the hematology department) will inevitably die. According to LLC "Cryocenter" (A.N. Gromyko), microbial air pollution in maternity hospitals in Moscow ranges from 104 to 105 CFU / m 3, and the last figure refers to the maternity hospital where homeless people are brought. The air of the Moscow metro contains approximately 700 CFU/m 3 . This is better than in the "conditionally clean" rooms of hospitals according to SanPiN. In clause 6.20 of the above SanPiN it is said “In sterile rooms, air is supplied by laminar or slightly turbulent jets (air velocity less than 0.15 m / s)”. This contradicts the laws of physics: at a speed of less than 0.2 m / s, the air flow cannot be laminar (unidirectional), and at less than 0.15 m / s it becomes not “weakly”, but highly turbulent (non-unidirectional).
SanPiN figures are not harmless, they are used to control facilities and examine projects by sanitary and epidemiological surveillance authorities. You can release advanced standards as much as you like, but as long as SanPiN 2.1.3.1375-03 exists, things will not budge. It's not just about mistakes. We are talking about the public danger of such documents. What is the reason for their appearance?
- Lack of knowledge of European norms and fundamentals of physics?
- knowledge, but
- intentionally worsening conditions in our hospitals?
- lobbying someone's interests (for example, manufacturers of inefficient air purification products)?
How does this relate to protecting public health and consumer rights? For us, consumers of healthcare services, such a picture is absolutely unacceptable. heavy before incurable diseases are leukemia and other blood diseases. Now there is a solution, and there is only one solution: bone marrow transplantation, then suppression of the body's immunity for the period of adaptation (1-2 months).
So that a person who is in a state of immunodeficiency does not die, he is placed in sterile air conditions (under laminar flow). This practice has been known around the world for decades. She also came to Russia. In 2005, two intensive care units for bone marrow transplantation were equipped in the Nizhny Novgorod Regional Children's Clinical Hospital. Chambers are made at the level of modern world practice.
This is the only way to save the doomed children. The patient's bed is located in the zone of unidirectional air flow (ISO class 5). But in the FGUZ "Center for Hygiene and Epidemiology of the Nizhny Novgorod Region" they staged an illiterate and ambitious paperwork, delaying the commissioning of the facility for six months. Do these employees understand that they may have unsaved children's lives on their conscience? The answer must be given to mothers by looking into their eyes.
Development of the national standard of Russia
An analysis of the experience of foreign colleagues made it possible to highlight several key issues, some of which caused a heated discussion when discussing the standard.
Room groups
Foreign standards mainly consider operational ones. Some standards deal with isolators and other spaces. There is no comprehensive systematization of premises for all purposes with a focus on the classification of cleanliness according to ISO. In the adopted standard, five groups of rooms are introduced depending on the risk of infection of the patient. Separately (group 5) isolated insulators and purulent operating rooms. Classification of premises is made taking into account risk factors.
Criteria for assessing air purity
What to take as a basis for assessing air purity:
- particles?
- microorganisms?
- this and that?
The development of norms in Western countries according to this criterion has its own logic. In the early stages, the purity of the air in hospitals was assessed only by the concentration of microorganisms. Then came the use of particle counting. Back in 1987, the French standard NFX 90-351 introduced air purity control for both particles and microorganisms. Particle counting with a laser particle counter allows you to quickly and in real time determine the concentration of particles, while for the incubation of microorganisms on nutrient medium it takes several days.
Next question: And what, in fact, is checked during the certification of clean rooms and ventilation systems? The quality of their work and the correctness of design decisions are checked. These factors are unambiguously evaluated by the concentration of particles, on which the number of microorganisms depends. Of course, microbial contamination depends on the cleanliness of walls, equipment, personnel, etc. But these factors relate to current work, to operation, and not to the assessment of engineering systems.
In this regard, Switzerland (SWKI 99-3) and Germany (VDI 2167) take a logical step forward: particulate air control installed. Recording of microorganisms remains a function of the epidemiological service of the hospital and is aimed at the current control of cleanliness. This idea was included in the project Russian standard. At this stage, it had to be abandoned due to the categorically negative position of the representatives of sanitary and epidemiological supervision.
Ultimately allowable norms for particles and microorganisms for various groups of premises are taken according to analogues with Western standards and based on our own experience. Particle classification corresponds to GOST ISO 14644-1.
Cleanroom states
GOST ISO 14644-1 distinguishes between three states of cleanrooms. In the constructed state, the execution of the series is checked technical requirements. The concentration of contaminants, as a rule, is not standardized. In the equipped state, the room is fully equipped with equipment, but there is no staff and there is no technological process(for hospitals - there is no medical staff and the patient).
In the operating state, all the processes provided for by the purpose of the premises are carried out in the premises. Production rules medicines— GMP (GOST R 52249-2004) provides for the control of contamination by particles both in the equipped state and in the operating state, and by microorganisms - only in the operated state. There is logic in this.
Emissions of contaminants from equipment and personnel during the production of medicines can be standardized and compliance with the standards can be ensured by technical and organizational measures. AT medical institution there is a non-standardized element - a sick one. It is impossible for him and the medical staff to dress in an ISO class 5 coverall and completely cover the entire surface of the body. Due to the fact that the sources of pollution in the operating state of the hospital premises cannot be controlled, it makes no sense to establish standards and certify the premises in the operated state, at least in terms of particles. This was understood by the developers of all foreign standards. We also included in GOST control of premises only in the equipped state.
Particle sizes
Cleanrooms were originally controlled for contamination with particles equal to or greater than 0.5 µm (≥ 0.5 µm). Then, based on specific applications, requirements began to appear for the concentration of particles ≥ 0.1 µm and ≥ 0.3 µm (microelectronics), ≥ 0.3 0.5 µm (production of drugs in addition to particles ≥ 0.5 µm ), etc. The analysis showed that in hospitals it makes no sense to follow the “0.5 and 5.0 µm” template, but it is enough to control particles ≥ 0.5 µm.
Unidirectional flow rate
It has already been noted above that SanPiN 2.1.3.3175-03, by setting the maximum permissible values for the speed of a unidirectional (laminar) flow of 0.15 m/s, violated the laws of physics. On the other hand, it is impossible to introduce the GMP norm of 0.45 m/s ±20% in medicine. This leads to discomfort, superficial dehydration of the wound, can injure it, etc. Therefore, for areas with unidirectional flow (operating rooms, intensive care wards), the speed is set from 0.24 to 0.3 m/s. This is the limit of the permissible, from which it is impossible to leave. The distribution of the modulus of air flow velocity in the area of the operating table for a real operating room in one of the hospitals, obtained by computer simulation, is shown below. It can be seen that at a low speed of the outgoing flow, it quickly turbulates and does not perform a useful function.
Dimensions of the zone with unidirectional airflow
A laminar zone with a "deaf" plane inside is useless. In the operating room of the Central Institute of Traumatology and Orthopedics (CITO), the author was operated on for an injury six years ago. It is known that a unidirectional air flow narrows at an angle of about 15% and what was in CITO does not make sense. The correct scheme (Klimed): It is no coincidence that Western standards provide for the size of a ceiling diffuser that creates a unidirectional flow of 3x3 m, without “deaf” surfaces inside. Exceptions are allowed for less critical operations.
Solutions for ventilation and air conditioning
These solutions comply with Western standards, are economical and efficient. Made some changes and simplifications without losing the meaning. For example, H14 filters (instead of H13) are used as final filters in operating rooms and intensive care units, which have the same cost, but are much more efficient.
Autonomous Air Cleaning Devices
Autonomous air cleaners are effective tool ensuring clean air (except for rooms of groups 1 and 2). They don't require high costs, allow for flexible decision-making and can be used en masse, especially in established hospitals. Presented on the market wide selection air cleaners. Not all of them are effective, some of them are harmful (they emit ozone). The main danger is the wrong choice of air cleaner. The Cleanroom Testing Laboratory conducts an experimental evaluation of air cleaners according to their intended use. Relying on reliable results is an important condition for fulfilling the requirements of GOST.
Test Methods
The manual SWKI 99-3 and the draft standard VDI 2167 give a method for testing operating rooms using dummies and aerosol generators (article by A. Brunner). The use of this technique in Russia is hardly justified. In a small country, one specialized laboratory can serve all hospitals. For Russia, this is unrealistic. From our point of view, it is not necessary. With the help of dummies, they work out standard solutions, which are laid down in the standard, and then serve as the basis for design. These standard solutions are being worked out in the conditions of the institute, which is done in Lucerne, Switzerland. In mass practice, standard solutions are applied directly. At the finished facility, tests are carried out for compliance with standards and the project. GOST R 52539-2006 gives a systematic test program for clean rooms in hospitals for all the necessary parameters.
Legionnaires' disease is a companion of old engineering systems
In 1976, an American Legion convention was held in a Philadelphia hotel. Of the 4,000 participants, 200 fell ill and 30 died. The cause was a microorganism species named Legionella pneumophila in connection with the mentioned event and numbering more than 40 varieties. The disease itself was named Legionnaires' disease. Symptoms of the disease appear 2-10 days after infection in the form of headache, pain in the limbs and throat, accompanied by fever.
The course of the disease is similar to ordinary pneumonia, and therefore it is often misdiagnosed as pneumonia. In Germany, with a population of about 80 million, about 10,000 people are officially estimated to suffer from Legionnaires' disease every year, but most cases remain unsolved. The risk category includes people with weakened immune systems, the elderly, young children, those with chronic diseases, and smokers.
The infection is transmitted by airborne droplets. The pathogen enters the indoor air from old ventilation and air conditioning systems, hot water systems, showers, etc. Legionella multiplies especially quickly in stagnant water at a temperature of 20 to 45 ° C. At 50°C, pasteurization occurs, and at 70°C, disinfection occurs. Dangerous sources are old large buildings (including hospitals and maternity hospitals) with ventilation systems and hot water supply. About measures to combat the disease - read on page 36 (Ed. note)
* Aspergillus, a common fungus that is usually harmless to humans, is a particular danger. But they pose a risk to the health of immunodeficient patients (for example, drug-induced immunosuppression after organ and tissue transplantation or patients with agranulocytosis). For such patients, inhalation of even small doses of Aspergillus spores can cause severe infectious diseases. In the first place here is a lung infection (pneumonia). In hospitals, there are frequent cases of infection associated with construction works or reconstruction. These cases are caused by the release of aspergillus spores from building materials during construction work, which requires the adoption of special protective measures(SWKI 99-3).
* Based on the article by M. Hartmann "Keep Legionella bugs at bay", Cleanroom Technology, March, 2006.