The topic of the current lesson will be the consideration of the processes occurring in quite specific, and not abstract, as in previous lessons, devices - heat engines. We will define such machines, describe their main components and the principle of operation. Also during this lesson, the question of finding efficiency - the efficiency of thermal engines, both real and maximum possible, will be considered.
Topic: Fundamentals of thermodynamics
Lesson: The principle of operation of a heat engine
The topic of the last lesson was the first law of thermodynamics, which set the relationship between a certain amount of heat that was transferred to a portion of a gas and the work done by this gas during expansion. And now it's time to say that this formula is of interest not only for some theoretical calculations, but also in quite practical application, because the work of a gas is nothing more than useful work, which we extract when using heat engines.
Definition. heat engine- a device in which the internal energy of the fuel is converted into mechanical work (Fig. 1).
Rice. 1. Various examples of heat engines (), ()
As can be seen from the figure, heat engines are any devices that work according to the above principle, and they range from incredibly simple to very complex in design.
Without exception, all heat engines are functionally divided into three components (see Fig. 2):
- Heater
- working body
- Fridge
Rice. 2. Functional diagram of a heat engine ()
The heater is the process of combustion of fuel, which, during combustion, transfers a large amount of heat to the gas, heating it to high temperatures. Hot gas, which is a working fluid, due to an increase in temperature and, consequently, pressure, expands, doing work. Of course, since there is always heat transfer with the engine casing, ambient air, etc., the work will not numerically equal the heat transferred - some of the energy goes to the refrigerator, which, as a rule, is the environment.
The easiest way is to imagine the process taking place in a simple cylinder under a movable piston (for example, the cylinder of an internal combustion engine). Naturally, for the engine to work and make sense, the process must occur cyclically, and not one-time. That is, after each expansion, the gas must return to its original position (Fig. 3).
Rice. 3. An example of the cyclic operation of a heat engine ()
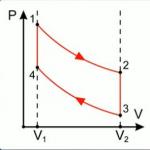
In order for the gas to return to its initial position, it is necessary to perform some work on it (the work of external forces). And since the work of the gas is equal to the work on the gas with the opposite sign, in order for the gas to perform a total positive work for the entire cycle (otherwise there would be no point in the engine), it is necessary that the work of external forces be less than the work of the gas. That is, the graph of the cyclic process in P-V coordinates should look like: a closed loop with a clockwise bypass. Under this condition, the work of the gas (in the section of the graph where the volume increases) is greater than the work on the gas (in the section where the volume decreases) (Fig. 4).

Rice. 4. An example of a graph of a process occurring in a heat engine
Since we are talking about a certain mechanism, it is imperative to say what its efficiency is.
Definition. Efficiency (coefficient of performance) of a heat engine- the ratio of useful work performed by the working fluid to the amount of heat transferred to the body from the heater.
If we take into account the conservation of energy: the energy that has departed from the heater does not disappear anywhere - part of it is removed in the form of work, the rest goes to the refrigerator:
We get:
![]()
This is an expression for efficiency in parts, if you need to get the efficiency value as a percentage, you need to multiply the resulting number by 100. The efficiency in the SI measurement system is a dimensionless value and, as can be seen from the formula, cannot be more than one (or 100).
It should also be said that this expression is called the real efficiency or the efficiency of a real heat engine (heat engine). If we assume that we somehow manage to completely get rid of the design flaws of the engine, then we will get an ideal engine, and its efficiency will be calculated according to the formula for the efficiency of an ideal heat engine. This formula was obtained by the French engineer Sadi Carnot (Fig. 5):
![]()
Modern realities involve the widespread operation of heat engines. Numerous attempts to replace them with electric motors have so far failed. The problems associated with the accumulation of electricity in autonomous systems are solved with great difficulty.
Still relevant are the problems of technology for the manufacture of electric power accumulators, taking into account their long-term use. The speed characteristics of electric vehicles are far from those of cars on internal combustion engines.
The first steps towards the creation of hybrid engines can significantly reduce harmful emissions in megacities, solving environmental problems.
A bit of history
The possibility of converting steam energy into motion energy was known in antiquity. 130 BC: Philosopher Heron of Alexandria presented to the audience a steam toy - aeolipil. A sphere filled with steam began to rotate under the action of jets emanating from it. This prototype of modern steam turbines did not find application in those days.
For many years and centuries, the development of the philosopher was considered only a fun toy. In 1629, the Italian D. Branchi created an active turbine. Steam set in motion a disk equipped with blades.
From that moment began the rapid development of steam engines.
heat engine

The conversion of fuel into energy for the movement of parts of machines and mechanisms is used in heat engines.
The main parts of machines: a heater (a system for obtaining energy from outside), a working fluid (performs a useful action), a refrigerator.
The heater is designed to ensure that the working fluid has accumulated a sufficient supply of internal energy to perform useful work. The refrigerator removes excess energy.
The main characteristic of efficiency is called the efficiency of thermal engines. This value shows what part of the energy spent on heating is spent on doing useful work. The higher the efficiency, the more profitable the operation of the machine, but this value cannot exceed 100%.
Efficiency calculation
Let the heater acquire from outside the energy equal to Q 1 . The working fluid did work A, while the energy given to the refrigerator was Q 2 .
Based on the definition, we calculate the efficiency:
η= A / Q 1 . We take into account that A \u003d Q 1 - Q 2.
From here, the efficiency of the heat engine, the formula of which has the form η = (Q 1 - Q 2) / Q 1 = 1 - Q 2 / Q 1, allows us to draw the following conclusions:
- Efficiency cannot exceed 1 (or 100%);
- to maximize this value, either an increase in the energy received from the heater or a decrease in the energy given to the refrigerator is necessary;
- an increase in the energy of the heater is achieved by changing the quality of the fuel;
- reducing the energy given to the refrigerator, make it possible to achieve the design features of the engines.

Ideal heat engine
Is it possible to create such an engine, the efficiency of which would be maximum (ideally, equal to 100%)? The French theoretical physicist and talented engineer Sadi Carnot tried to find the answer to this question. In 1824, his theoretical calculations about the processes occurring in gases were made public.
The main idea behind an ideal machine is to carry out reversible processes with an ideal gas. We start with the expansion of the gas isothermally at a temperature T 1 . The amount of heat required for this is Q 1. After the gas expands without heat exchange. Having reached the temperature T 2, the gas is compressed isothermally, transferring the energy Q 2 to the refrigerator. The return of the gas to its original state is adiabatic.
The efficiency of an ideal Carnot heat engine, when accurately calculated, is equal to the ratio of the temperature difference between the heating and cooling devices to the temperature that the heater has. It looks like this: η=(T 1 - T 2)/ T 1.
The possible efficiency of a heat engine, the formula of which is: η= 1 - T 2 / T 1 , depends only on the temperature of the heater and cooler and cannot be more than 100%.
Moreover, this ratio allows us to prove that the efficiency of heat engines can be equal to unity only when the refrigerator reaches temperatures. As you know, this value is unattainable.
Carnot's theoretical calculations make it possible to determine the maximum efficiency of a heat engine of any design.
The theorem proved by Carnot is as follows. An arbitrary heat engine under no circumstances is capable of having a coefficient of efficiency greater than the similar value of the efficiency of an ideal heat engine.

Example of problem solving
Example 1 What is the efficiency of an ideal heat engine if the heater temperature is 800°C and the refrigerator temperature is 500°C lower?
T 1 \u003d 800 o C \u003d 1073 K, ∆T \u003d 500 o C \u003d 500 K, η -?
By definition: η=(T 1 - T 2)/ T 1.
We are not given the temperature of the refrigerator, but ∆T = (T 1 - T 2), from here:
η \u003d ∆T / T 1 \u003d 500 K / 1073 K \u003d 0.46.
Answer: efficiency = 46%.
Example 2 Determine the efficiency of an ideal heat engine if 650 J of useful work is performed due to the acquired one kilojoule of heater energy. What is the temperature of the heat engine heater if the coolant temperature is 400 K?
Q 1 \u003d 1 kJ \u003d 1000 J, A \u003d 650 J, T 2 \u003d 400 K, η -?, T 1 \u003d?
In this problem, we are talking about a thermal installation, the efficiency of which can be calculated by the formula:
To determine the temperature of the heater, we use the formula for the efficiency of an ideal heat engine:
η \u003d (T 1 - T 2) / T 1 \u003d 1 - T 2 / T 1.
After performing mathematical transformations, we get:
T 1 \u003d T 2 / (1- η).
T 1 \u003d T 2 / (1- A / Q 1).
Let's calculate:
η= 650 J / 1000 J = 0.65.
T 1 \u003d 400 K / (1- 650 J / 1000 J) \u003d 1142.8 K.
Answer: η \u003d 65%, T 1 \u003d 1142.8 K.
Real conditions
The ideal heat engine is designed with ideal processes in mind. Work is done only in isothermal processes, its value is defined as the area bounded by the Carnot cycle graph.

In fact, it is impossible to create conditions for the process of changing the state of a gas without accompanying changes in temperature. There are no materials that would exclude heat exchange with surrounding objects. The adiabatic process is no longer possible. In the case of heat transfer, the temperature of the gas must necessarily change.
The efficiency of heat engines created in real conditions differ significantly from the efficiency of ideal engines. Note that the processes in real engines are so fast that the variation in the internal thermal energy of the working substance in the process of changing its volume cannot be compensated by the influx of heat from the heater and return to the cooler.
Other heat engines
Real engines operate on different cycles:
- Otto cycle: the process at a constant volume changes adiabatically, creating a closed cycle;
- Diesel cycle: isobar, adiabat, isochor, adiabat;
- the process occurring at constant pressure is replaced by an adiabatic one, closing the cycle.
It is not possible to create equilibrium processes in real engines (to bring them closer to ideal ones) under the conditions of modern technology. The efficiency of thermal engines is much lower, even taking into account the same temperature regimes as in an ideal thermal installation.
But you should not reduce the role of the efficiency calculation formula, since it is it that becomes the starting point in the process of working to increase the efficiency of real engines.
Ways to change efficiency
When comparing ideal and real heat engines, it is worth noting that the temperature of the refrigerator of the latter cannot be any. Usually the atmosphere is considered to be a refrigerator. The temperature of the atmosphere can be taken only in approximate calculations. Experience shows that the temperature of the coolant is equal to the temperature of the exhaust gases in the engines, as is the case in internal combustion engines (abbreviated internal combustion engines).

ICE is the most common heat engine in our world. The efficiency of a heat engine in this case depends on the temperature created by the burning fuel. An essential difference between an internal combustion engine and steam engines is the merging of the functions of the heater and the working fluid of the device in the air-fuel mixture. Burning, the mixture creates pressure on the moving parts of the engine.
An increase in the temperature of the working gases is achieved by significantly changing the properties of the fuel. Unfortunately, it is not possible to do this indefinitely. Any material from which the combustion chamber of an engine is made has its own melting point. The heat resistance of such materials is the main characteristic of the engine, as well as the ability to significantly affect the efficiency.
Motor efficiency values
If we consider the temperature of the working steam at the inlet of which is 800 K, and the exhaust gas is 300 K, then the efficiency of this machine is 62%. In reality, this value does not exceed 40%. Such a decrease occurs due to heat losses during heating of the turbine housing.

The highest value of internal combustion does not exceed 44%. Increasing this value is a matter of the near future. Changing the properties of materials, fuels is a problem that the best minds of mankind are working on.
Since ancient times, people have tried to convert energy into mechanical work. They converted the kinetic energy of the wind, the potential energy of water, etc. Starting from the 18th century, machines began to appear that convert the internal energy of fuel into work. Such machines worked thanks to heat engines.
A heat engine is a device that converts thermal energy into mechanical work due to expansion (most often gases) from high temperature.
Any heat engines have components:
- A heating element. A body with a high temperature relative to the environment.
- working body. Since expansion provides the job, this element must expand well. As a rule, gas or steam is used.
- cooler. Body with low temperature.
The working fluid receives thermal energy from the heater. As a result, it begins to expand and do work. In order for the system to perform work again, it must be returned to its original state. Therefore, the working fluid is cooled, that is, excess thermal energy is, as it were, discharged into the cooling element. And the system comes to its original state, then the process repeats again.
Efficiency calculation
To calculate the efficiency, we introduce the following notation:
Q 1 - The amount of heat received from the heating element
A’– Work done by the working body
Q 2 - The amount of heat received by the working fluid from the cooler
In the process of cooling, the body transfers heat, so Q 2< 0.
The operation of such a device is a cyclic process. This means that after a complete cycle, the internal energy will return to its original state. Then, according to the first law of thermodynamics, the work done by the working fluid will be equal to the difference between the amount of heat received from the heater and the heat received from the cooler:
Q 2 is a negative value, so it is taken modulo
Efficiency is expressed as the ratio of useful work to the total work that the system has performed. In this case, the total work will be equal to the amount of heat that is spent on heating the working fluid. All expended energy is expressed through Q 1 .
The main significance of the formula (5.12.2) obtained by Carnot for the efficiency of an ideal machine is that it determines the maximum possible efficiency of any heat engine.
Carnot proved, based on the second law of thermodynamics*, the following theorem: any real heat engine operating with a temperature heaterT 1 and refrigerator temperatureT 2 , cannot have an efficiency exceeding the efficiency of an ideal heat engine.
* Carnot actually established the second law of thermodynamics before Clausius and Kelvin, when the first law of thermodynamics had not yet been formulated rigorously.
Consider first a heat engine operating on a reversible cycle with a real gas. The cycle can be any, it is only important that the temperatures of the heater and refrigerator are T 1 And T 2 .
Let us assume that the efficiency of another heat engine (not operating according to the Carnot cycle) η ’ > η . The machines work with a common heater and a common cooler. Let the Carnot machine work in the reverse cycle (like a refrigeration machine), and the other machine in the forward cycle (Fig. 5.18). The heat engine performs work equal, according to formulas (5.12.3) and (5.12.5):

The refrigeration machine can always be designed so that it takes the amount of heat from the refrigerator Q 2
= | |
|
Then, according to formula (5.12.7), work will be performed on it
 (5.12.12)
(5.12.12)
Since by condition η" > η , That A" > A. Therefore, the heat engine can drive the refrigeration engine, and there will still be an excess of work. This excess work is done at the expense of heat taken from one source. After all, heat is not transferred to the refrigerator under the action of two machines at once. But this contradicts the second law of thermodynamics.
If we assume that η > η ", then you can make another machine work in a reverse cycle, and Carnot's machine in a straight line. We again come to a contradiction with the second law of thermodynamics. Therefore, two machines operating on reversible cycles have the same efficiency: η " = η .
It is a different matter if the second machine operates in an irreversible cycle. If we allow η "
>
η ,
then we again come to a contradiction with the second law of thermodynamics. However, the assumption m|"< г| не противоречит второму закону
термодинамики, так как необратимая
тепловая машина не может работать как
холодильная машина. Следовательно, КПД
любой тепловой машины η"
≤ η, or 
This is the main result:
 (5.12.13)
(5.12.13)
Efficiency of real heat engines
Formula (5.12.13) gives the theoretical limit for the maximum efficiency of heat engines. It shows that the heat engine is more efficient, the higher the temperature of the heater and the lower the temperature of the refrigerator. Only when the refrigerator temperature is equal to absolute zero, η = 1.
But the temperature of the refrigerator practically cannot be much lower than the ambient temperature. You can increase the temperature of the heater. However, any material (solid) has limited heat resistance, or heat resistance. When heated, it gradually loses its elastic properties, and melts at a sufficiently high temperature.
Now the main efforts of engineers are aimed at increasing the efficiency of engines by reducing the friction of their parts, fuel losses due to its incomplete combustion, etc. The real opportunities for increasing the efficiency are still large here. So, for a steam turbine, the initial and final steam temperatures are approximately as follows: T 1 = 800 K and T 2 = 300 K. At these temperatures, the maximum value of the efficiency is:

The actual value of the efficiency due to various kinds of energy losses is approximately 40%. The maximum efficiency - about 44% - have internal combustion engines.
The efficiency of any heat engine cannot exceed the maximum possible value  ,
where T 1
-
absolute temperature of the heater, and T 2
-
absolute temperature of the refrigerator.
,
where T 1
-
absolute temperature of the heater, and T 2
-
absolute temperature of the refrigerator.
Increasing the efficiency of heat engines and bringing it closer to the maximum possible- the most important technical challenge.
In the theoretical model of a heat engine, three bodies are considered: heater, working body And fridge.
Heater - a thermal reservoir (large body), the temperature of which is constant.
In each cycle of engine operation, the working fluid receives a certain amount of heat from the heater, expands and performs mechanical work. The transfer of part of the energy received from the heater to the refrigerator is necessary to return the working fluid to its original state.
Since the model assumes that the temperature of the heater and refrigerator does not change during the operation of the heat engine, then at the end of the cycle: heating-expansion-cooling-compression of the working fluid, it is considered that the machine returns to its original state.
For each cycle, based on the first law of thermodynamics, we can write that the amount of heat Q load received from the heater, amount of heat | Q cool |, given to the refrigerator, and the work done by the working body A are related to each other by:
A = Q load – | Q cold|.
In real technical devices, which are called heat engines, the working fluid is heated by the heat released during the combustion of fuel. So, in a steam turbine of a power plant, the heater is a furnace with hot coal. In an internal combustion engine (ICE), combustion products can be considered a heater, and excess air can be considered a working fluid. As a refrigerator, they use the air of the atmosphere or water from natural sources.
Efficiency of a heat engine (machine)
Heat engine efficiency (efficiency) is the ratio of the work done by the engine to the amount of heat received from the heater:
![]()
The efficiency of any heat engine is less than one and is expressed as a percentage. The impossibility of converting the entire amount of heat received from the heater into mechanical work is the price to pay for the need to organize a cyclic process and follows from the second law of thermodynamics.
In real heat engines, the efficiency is determined by the experimental mechanical power N engine and the amount of fuel burned per unit time. So, if in time t mass fuel burned m and specific heat of combustion q, That
For vehicles, the reference characteristic is often the volume V fuel burned on the way s at mechanical engine power N and at speed. In this case, taking into account the density r of the fuel, we can write a formula for calculating the efficiency:
Second law of thermodynamics
There are several formulations second law of thermodynamics. One of them says that a heat engine is impossible, which would do work only due to a heat source, i.e. without refrigerator. The world ocean could serve for it as a practically inexhaustible source of internal energy (Wilhelm Friedrich Ostwald, 1901).
Other formulations of the second law of thermodynamics are equivalent to this one.
Clausius' formulation(1850): a process is impossible in which heat would spontaneously transfer from less heated bodies to more heated bodies.
Thomson's formulation(1851): a circular process is impossible, the only result of which would be the production of work by reducing the internal energy of the thermal reservoir.
Clausius' formulation(1865): all spontaneous processes in a closed non-equilibrium system occur in such a direction in which the entropy of the system increases; in a state of thermal equilibrium, it is maximum and constant.
Boltzmann's formulation(1877): a closed system of many particles spontaneously passes from a more ordered state to a less ordered one. The spontaneous exit of the system from the equilibrium position is impossible. Boltzmann introduced a quantitative measure of disorder in a system consisting of many bodies - entropy.
Efficiency of a heat engine with an ideal gas as a working fluid
If the model of the working fluid in a heat engine is given (for example, an ideal gas), then it is possible to calculate the change in the thermodynamic parameters of the working fluid during expansion and contraction. This allows you to calculate the efficiency of a heat engine based on the laws of thermodynamics.
The figure shows the cycles for which the efficiency can be calculated if the working fluid is an ideal gas and the parameters are set at the points of transition of one thermodynamic process to another.
|
|
Isobaric-isochoric |
|
Isochoric-adiabatic |
|
Isobaric-adiabatic |
|
Isobaric-isochoric-isothermal |
|
|
Isobaric-isochoric-linear |
Carnot cycle. Efficiency of an ideal heat engine

The highest efficiency at given heater temperatures T heating and refrigerator T cold has a heat engine where the working fluid expands and contracts along Carnot cycle(Fig. 2), the graph of which consists of two isotherms (2–3 and 4–1) and two adiabats (3–4 and 1–2).
Carnot's theorem proves that the efficiency of such an engine does not depend on the working fluid used, so it can be calculated using the thermodynamic relations for an ideal gas:
![]()
Environmental consequences of heat engines
The intensive use of heat engines in transport and energy (thermal and nuclear power plants) significantly affects the Earth's biosphere. Although there are scientific disputes about the mechanisms of the influence of human activity on the Earth's climate, many scientists point out the factors due to which such an influence can occur:
- The greenhouse effect is an increase in the concentration of carbon dioxide (a product of combustion in the heaters of thermal machines) in the atmosphere. Carbon dioxide transmits visible and ultraviolet radiation from the Sun, but absorbs infrared radiation from the Earth. This leads to an increase in the temperature of the lower layers of the atmosphere, an increase in hurricane winds and global ice melting.
- Direct impact of toxic exhaust gases on wildlife (carcinogens, smog, acid rain from combustion by-products).
- Destruction of the ozone layer during aircraft flights and rocket launches. The ozone of the upper atmosphere protects all life on Earth from excess ultraviolet radiation from the Sun.
The way out of the emerging ecological crisis lies in increasing the efficiency of heat engines (the efficiency of modern heat engines rarely exceeds 30%); use of serviceable engines and neutralizers of harmful exhaust gases; use of alternative energy sources (solar batteries and heaters) and alternative means of transport (bicycles, etc.).