Lesson topic: gas laws. Laws of hydrostatics and hydrodynamics.
A gas is one of the aggregate states of a substance in which its particles move freely, evenly filling the space available to them. They exert pressure on the shell that limits this space. The density of a gas at normal pressure is several orders of magnitude less than the density of a liquid.
Laws of gas dynamics
- Boyle-Mariotte's law (Isothermal process)
- Charles' law (Isochoric process) and Gay-Lussac (Isobaric process)
- Dalton's Law
- Henry's law
- Pascal's law
- Law of Archimedes
- Euler-Bernoulli law
Boyle-Mariotte's law (Isothermal process)
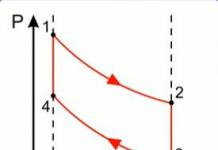
- For a given mass of gas M at a constant temperature T, its volume V is inversely proportional to pressure P: PV \u003d const, P 1 V 1 \u003d P 2 V 2, P 1 and P 2 - initial and final pressure values, V 1 and V 2 - initial and final pressure value.
- Conclusion - How many times the pressure increases, so many times the volume decreases.
- Using this law, you can understand how many times with increasing depth the air consumption for breathing of an underwater swimmer increases, and also calculate the time spent under water.
- Example: V cylinder = 15l, P cylinder = 200, Bar V lung = 5l, D depth = 40m How long will the cylinder last at this depth? If a person takes 6 breaths per minute? 15x200 = 3000l of air in the tank, 5x6=30l/min - air consumption per minute on the surface. At a depth of 40m, P abs =5 bar, 30x5=150 l/min at a depth. 3000/150= 20min. Answer: enough air for 30 minutes.
Charles' law (Isochoric process) and Gay-Lussac (Isobaric process)
- For a given mass of gas M at constant volumeV pressure is directly proportional to the change in its absolute temperature T: P 1 xT 1 = P 2 xT 2
- For a given mass of gas M at constant pressure P the volume of gas changes in direct proportion to the change in absolute temperature T: V 1 xT 1 \u003d V 2 xT 2
- Absolute temperature is expressed in degrees Kelvin. 0°C=273°K, 10°C=283°K, -10°C=263°K
- Example: Assume that the cylinder was filled with compressed air at a pressure of 200 bar, after which the temperature rose to 70°C. What is the air pressure inside the balloon? P 1 \u003d 200, T 1 \u003d 273, P 2 \u003d?, T 2 \u003d 273 + 70 \u003d 343, P 1 xT 1 \u003d P 2 xT 2, P 2 \u003d P 2 xT 2 / T 1 \u003d 200 × 343/273 = 251 bar
Dalton's Law
- The absolute pressure of a mixture of gases is equal to the sum of the partial (partial) pressures of the individual gases that make up the mixture.
- The partial gas pressure P g is proportional to the percentage n of the given gas and the absolute pressure P abs of the gas mixture and is determined by the formula: P g = P abs n/100. This law can be illustrated by comparing a mixture of gases in a closed volume with a set of weights of different weights placed on a balance. Obviously, each of the weights will exert pressure on the scales regardless of the presence of other weights on it.
Henry's law
- The amount of gas dissolved in a liquid is directly proportional to its partial pressure. If the partial pressure of a gas doubles, then the amount of dissolved gas doubles. When a swimmer dives, P abs increases, therefore the amount of gas inhaled by the swimmer becomes larger and, accordingly, it dissolves in the blood in a larger amount. When you ascend, the pressure decreases and the gas dissolved in the blood comes out in the form of bubbles, as when opening a bottle of carbonated water. This mechanism underlies DCS.
Laws of hydrostatics and hydrodynamics
For water, as well as for gases, due to their fluidity, Pascal's law is fulfilled, which determines the ability of these media to transmit pressure. For a body immersed in a liquid, the law of Archimedes is fulfilled, due to the action on the surface of the body of pressure created by the liquid due to its weight (i.e., the action of gravity). For moving liquids and gases, the Euler-Bernoulli law is valid.
Pascal's Law
The pressure on the surface of a liquid (or gas), produced by external forces, is transmitted by the liquid (or gas) equally in all directions.
The action of this law underlies the operation of all kinds of hydraulic apparatus and devices, including scuba gear (cylinders - reducer - breathing machine)
Law of Archimedes
Any body immersed in a liquid (or gas) is acted upon by this liquid (or gas) by a force directed upwards, applied to the center of gravity of the displaced volume and equal in magnitude to the weight of the liquid (or gas) displaced by the body.
Q= yV
at – specific gravity of the liquid;
V- the volume of water displaced by the body (immersed volume).
Archimedes' law defines such qualities of bodies immersed in a liquid as buoyancy and stability.
Euler-Bernoulli law
The pressure of the flowing liquid (or gas) is greater in those sections of the flow in which the speed of movement is less, and vice versa, in those sections in which the speed of movement is greater, the pressure is less .
Accurate calculation of diving air is the second most important factor after the perfect technical condition of the equipment. Since this task has been standing since the very moment of the invention of scuba gear, special methods for calculating the required volume of air have long been developed. The volume of air required by one diver per minute is taken as a basis and then the resulting value is divided by the volume of gas in the cylinder.
These calculations are complicated by the fact that air consumption depends on physical activity. With quiet swimming, it is much less than with intensive finning. Another factor that is also always taken into account is the depth of immersion. The deeper the depth, the more pressure you need to supply air. All factors taken into account can be represented as a list:
- The volume of the balloon.
- The pressure in the balloon.
- Air consumption per minute (referred to as RMV)
- Immersion depth.
The first two parameters can be very precise. Their accuracy depends only on how they correspond to the indicated volume, as well as how accurately the valve on the pump that was used for filling is adjusted. The compressor is switched off at the end of refueling by a pressure sensor. It is from that that is responsible for the fact that the volume of air in the cylinder exactly corresponds to the declared one.
The hardest thing to calculate is RMV. Accurate data can only be obtained empirically. This is exactly what they do when they train divers. The student memorizes the readings of the pressure gauge in various modes of diving, drifting with the flow, lifting or standing still. Further, on the basis of the received data, an individual RMV indicator is displayed. The data is recorded in the form of a table with three columns: dive time and depth, and pressure in the cylinder on a manometer. Having recalculated the pressure in the cylinder by the volume (you just need to multiply the indicators), we will get the exact value of air consumption per minute and derive corrections for load and depth.
If there is no time for such measurements, which require trial dives with an instructor, then general indicators are taken. They are calculated with some margin, which is necessary to cover all individual features. So the air consumption at the surface by a diver weighing 80 kg is 20 - 25 l/min. (Actually, somewhat less - 16 - 22 liters). Women have even less air intake. The next step is to correct for depth. With increasing depth of immersion, the volume of air required increases very quickly. At 50 meters (maximum depth for amateur diving), you need almost twice as much (about 40 l / min.).
For different mixtures, the maximum inhalation pressure is different. For oxygen, it is only 1.3 - 1.4 atm. For this reason, deep-sea diving requires special mixtures. When compiling, they try to make the oxygen content in them slightly different from the natural one in ordinary air. The nitrogen content in the deep-water mixture is also reduced, since if ordinary air is used, nitrogen anesthesia begins already at 30 meters. For the deepest dives, a helium-oxygen mixture is optimal. In amateur diving, it is almost never used. Filling cylinders with helium is difficult because it is characterized by ultra-high permeability, however, in a mixture with oxygen, this disadvantage is almost leveled.
When using clean air, where the cylinder was filled is also important. There is only one main requirement. Air purity is essential. Therefore, it is better with an electric drive. Then the risk of getting carbon monoxide and excess carbon dioxide is minimal. It is optimal that the filling of cylinders is carried out in an environmentally friendly place, for example, on the seashore or in the countryside.
Tasks
Solution.
Solution.
Examples
20 l oxygen cylinder is pressurized
10 MPa at 15 ºС. After some of the oxygen was used up, the pressure dropped to 7.6 MPa, and the temperature dropped to 10 ºС.
Determine the mass of oxygen consumed.
From the characteristic equation (2.5)
Therefore, before the consumption of oxygen, its mass consisted
![]() kg,
kg,
and after spending
![]() kg.
kg.
So the oxygen consumption
ΔM \u003d M 1 -M 2\u003d 2.673 - 2.067 \u003d 0.606 kg.
Determine the density and specific volume of carbon monoxide SO at a pressure of 0.1 MPa at a temperature of 27 ºС.
The specific volume is determined from the characteristic equation (2.6)
![]()
![]() m 3 /kg .
m 3 /kg .
Density of carbon monoxide (1.2)
![]() kg / m 3.
kg / m 3.
A cylinder with a movable piston contains oxygen at
t= 80 ºС and rarefaction (vacuum) equal to 427 hPa. At a constant temperature, oxygen is compressed to excess pressure
p est= 1.2 MPa. barometric pressure IN= 933 hPa.
By how much will the volume of oxygen decrease?
Answer:V 1 / V 2 = 22,96.
In a room with an area of 35 m 2 and a height of 3.1 m, air is at t= 23 ºС and barometric pressure IN= 973 hPa.
How much air will penetrate from the street into the room if the barometric pressure increases to IN= 1013 hPa. The air temperature remains constant.
Answer:M = 5.1 kg .
A vessel with a volume of 5 m 3 contains air at barometric pressure IN= 0.1 MPa and temperature 300 ºС. Then the air is pumped out until a vacuum pressure of 80 kPa is formed in the vessel. The air temperature after pumping out remains the same.
How much air is pumped out? What will be the pressure in the vessel after pumping out, if the remaining air is cooled to a temperature t= 20 ºС?
Answer: pumped out 2.43 kg of air. After cooling the air, the pressure will be 10.3 kPa.
130,000 m 3 /h of air at a temperature of 30 ºС is supplied to the air heater of the steam boiler by a fan.
Determine the volumetric air flow at the outlet of the air heater if it is heated to 400 ºС at constant pressure.
Answer:V= 288700 m 3 / h.
How many times will the density of the gas in the vessel change if, at a constant temperature, the pressure gauge reading decreases from p 1= 1.8 MPa up to p 2= 0.3 MPa?
Barometric pressure is taken equal to 0.1 MPa.
Answer:
In a vessel with a volume of 0.5 m 3 there is air at a pressure of 0.2 MPa and a temperature of 20 ºС.
How much air must be pumped out of the vessel so that the vacuum in it is 56 kPa, provided that the temperature in the vessel does not change? Atmospheric pressure according to a mercury barometer is 102.4 kPa at a temperature of mercury in it equal to 18 ºС. The vacuum in the vessel was measured with a mercury vacuum gauge at a mercury temperature of 20 ºС.
Answer: M= 1.527 kg.
Often it is necessary to solve problems in which not individual gases are considered, but their mixtures. When mixing chemically non-interacting gases having different pressures and temperatures, it is usually necessary to determine the final state of the mixture. In this case, two cases are distinguished (Table 1).
Table 1
Gas mixing*
| Temperature, K | Pressure, Pa | Volume, m 3 (volume flow, m 3 / h) | |
| Gas mixing at V=const |  | ||
| Mixing of gas streams** |  | ||
| * - all equations related to the mixing of gases are derived under the condition that there is no heat exchange with the environment; ** - if mass costs ( M 1, M 2, ... M n, kg/h) of mixing flows are equal. |
Here k i is the ratio of heat capacities of gases (see formula (4.2)).
Gas mixtures are understood as a mechanical mixture of several gases that do not chemically interact with each other. The composition of the gas mixture is determined by the amount of each of the gases included in the mixture, and can be set by mass m i or voluminous r i shares:
m i = M i / M; r i = V i / V, (3.1)
Where M i- weight i-th component,
Vi- partial or reduced volume i- th component;
M, V are the mass and volume of the whole mixture, respectively.
It's obvious that
M 1 + M 2 + ... + M n \u003d M; m 1 + m 2 +…+m n = 1, (3.2)
V 1 + V 2 +…+ V n = V ;r 1 + r 2 +…+r n = 1, (3.3)
The relationship between the pressure of the gas mixture R and partial pressure of individual components p i included in the mixture is set dalton's law
Compression of air in a vessel immersed in water
Consider the following situation. An empty open glass bottle is immersed in water to a depth h.
1. Explain why when a bottle is immersed upside down, air bubbles out of it and the bottle fills with water (Fig. 46.1).
2. Why does the bottle immediately sink?
3. Explain why when the bottle is immersed upside down, air does not escape from it (Fig. 46.2).
4. Explain why when a bottle is immersed upside down, the volume of air in it decreases with increasing depth.
Let us denote the density of water ρ in, the internal volume of the bottle V 0, the volume of air contained in it V air, atmospheric pressure p a. We assume that the temperature of the air in the bottle remains constant.
5. Explain why when the bottle is immersed to a depth h, the equation is true
V air (p a + ρ in gh) \u003d V 0 p a. (1)
6. How many times will the volume of air in the bottle decrease when it is immersed to a depth of 10 m?
7. How does the Archimedes force acting on the air bottle change with increasing depth?
8. Explain why in this case, when finding the Archimedes force, the volume of a body immersed in water must be considered equal to the total volume of glass and air in the bottle.
At a certain depth of immersion, the force of Archimedes will become equal to the force of gravity. When diving to even greater depths, the force of Archimedes will be less than the force of gravity, so the bottle of air will begin to sink.
Let us pose the question: is it possible to neglect the force of gravity acting on the air, in comparison with the force of gravity acting on the bottle?
9. How many times is the mass of air contained in a half-liter bottle less than the mass of the bottle? Take the mass of the bottle equal to 0.5 kg; air density at 20 ºС is approximately equal to 1.2 kg/m 3 .
So, we see that the mass of air in the bottle can be neglected with good accuracy compared to the mass of the bottle.
Let us denote the glass density as ρ c and the glass volume as V c.
10. Explain why, when a bottle of air is completely submerged in water and is in equilibrium, the following equation is true:
ρ with V with g = ρ in g(V air + V c). (2)
Equations (1) and (2) can be considered as a system of two equations with two unknowns. For example, if the values of all the quantities included in these equations are known, except for Vair and h, they can be found using these equations.
11. An open bottle containing air at atmospheric pressure is lowered upside down into the water. Bottle capacity 0.5 l, glass volume 0.2 l, glass density 2.5 times that of water, atmospheric pressure 100 kPa.
a) What is the volume of air in the bottle when the bottle immersed in water is in equilibrium?
b) At what depth will the bottle be?
In the considered situation, the mass of air can be neglected, because at a pressure close to atmospheric, the density of air is much less than the density of water and solids.
But in cases when it comes to lifting loads from great depths with compressed air, the mass of compressed air can be significant.
Consider an example.
12. Explorers of the ocean depths found a sunken treasure chest at a depth of 1 km. The weight of the chest is 2.5 tons, the volume is 1 m 3. The chest was tied with a cable to a strong empty waterproof bag and air was pumped into the bag until it began to float along with the chest. To simplify the calculations, we will take the density of sea water equal to the density of fresh water. We will assume that water is incompressible, and the volume of the bag shell is negligible. The water temperature at great depths can be considered close to 0 ºС.
a) Should atmospheric pressure be taken into account to determine the air pressure in the bag?
b) We denote ρ the density of water, m c and m into the mass of the chest and the mass of air in the bag, V c and V into the volume of the chest and the volume of air at the beginning of the ascent, M v is the molar mass of air, T is the absolute temperature of the water. Write down a system of two equations with two unknowns (m in and V in), assuming that atmospheric pressure can be neglected.
c) What is the volume of air in the bag at the moment when the bag with the chest began to float?
d) What is the mass of air in the bag when the bag with the chest began to float?
e) Is it possible to keep the air out of the bag until the bag with the chest floats to the surface?
Air in a mercury tube
Air is contained in a glass tube sealed at one end. This air is separated from atmospheric air by a column of mercury with a length of l rt (Fig. 46.3). 
Let us consider how the length of the part of the tube filled with air depends on the position of the tube and the air temperature in it. We will assume that the length of the tube is large enough so that mercury does not pour out of the tube in any position.
We denote the atmospheric pressure p a, the density of mercury ρ RT, and the length of the air-filled part of the tube, when it is located horizontally, denote l 0 .
Let us first assume that the temperature of the air in the tube is constant.
13. Write an equation that relates the values \u200b\u200bof l rt, l 0 and the length l of the air-filled part of the tube when it is located:
a) vertically open end up;
b) vertically open end down.
14. At the initial moment, the tube is located with the open end down. When it was turned upside down, the length of the air-filled part of the tube was reduced by 10%. What is the length of a column of mercury if atmospheric pressure is 760 mm Hg. Art.?
Let us now consider the case when the air temperature in the cabin changes.
15. At the initial moment, the tube with air and a column of mercury is located horizontally. When it was lowered into boiling water with the open end up, the length of the air-filled part of the tube increased by 20%. What is the initial temperature of the air in the tube if the length of the mercury column is 5 cm? Atmospheric pressure is 760 mm Hg. Art.
2. Two gases in a cylinder with a piston or baffle
The cylinder is horizontal
Let us first consider the case when a cylinder with different gases is located horizontally (in Figure 46.4, different gases are schematically indicated by different colors). In this case, the weight of the piston can be ignored. 
The piston can have various properties that must be taken into account when solving problems.
16. What can be said about the pressure and temperature of two gases separated by a piston if it:
a) heat-conducting and can move without friction?
b) does not conduct heat, but can move without friction?
c) heat-conducting, but friction between the piston and the walls of the vessel should be taken into account?
17. In a horizontally located cylinder with a piston on opposite sides of the piston are hydrogen and oxygen.
a) What is the relationship between the volumes of gases and the amount of substance in them, if the piston is movable and heat-conducting?
b) What is the relationship between the volumes and masses of gases in this case?
c) How are the volumes, masses and temperatures of gases related if the piston is movable but does not conduct heat?
If it is said that the vessel is divided not by a piston, but by a partition, then it is understood that the volumes of the parts of the vessel remain constant. The partition can also have different properties.
18. What can be said about the temperature and partial pressure of two gases separated by a partition if it:
a) thermally conductive?
b) porous (this usually means that the molecules of one gas can penetrate the partition, but the molecules of another gas cannot)?
19. A heat-insulated vessel is divided by a porous partition into two equal parts. At the initial moment, there are 2 moles of helium on the left side of the vessel, and 1 mole of argon on the right. The initial temperature of helium is 300 K, and the initial temperature of argon is 600 K. Helium atoms can freely penetrate through the pores in the partition, but argon atoms cannot.
a) Does it matter whether the baffle conducts heat or not?
b) The atoms of which gas at the initial moment have a greater average kinetic energy? How many times greater?
c) The internal energy of which gas is greater at the initial moment? How many times more?
d) Explain why the average kinetic energies of atoms of different gases are equal after reaching thermal equilibrium.
e) What will be the temperature in the vessel at thermal equilibrium?
f) How many times will the average kinetic energy of helium atoms at thermal equilibrium be greater than their average kinetic energy in the initial state?
g) How will the helium pressure in the left side of the vessel change compared to the initial one after equilibrium is established?
h) How will the argon pressure change compared to the initial one after equilibrium is established?
i) The pressure in which part of the vessel will be greater after equilibrium is established? How many times more?
The cylinder is vertical
If the cylinder is located vertically (Fig. 46.5), then the weight of the piston must be taken into account, which presses on the gas located at the bottom of the cylinder. Because of this, the pressure at the bottom of the cylinder is greater than at the top. Consider an example.
20. A vertically located cylindrical vessel of height l is divided by a movable piston into two parts. In the upper part with a height l in there are ν moles of helium, and in the lower part with a height l n - the same number of moles of hydrogen. The temperature of the gases remains always equal to T. The mass of the piston m, the area S, the thickness of the piston can be neglected compared to the height of the vessel.
a) Express the pressure in each part of the vessel in terms of other quantities. Does the type of gas in the parts of the vessel matter for this?
b) Write an equation relating the pressure of gases in each part of the vessel with the mass of the piston and its area.
c) What is the mass of the piston if l \u003d 50 cm, ν \u003d 0.22 mol, T \u003d 361 K, l in \u003d 30 cm?
Clue. Use the ideal gas equation of state.
Lift force of the balloon
A balloon (Fig. 46.6) can be in equilibrium in the air only if the Archimedes force acting on it from the side of the air is equal in absolute value to the total gravity acting on the balloon and the load suspended from it:
F A \u003d F t.sh + F t.gr. (3)

In the case of a balloon, the Archimedes force is equal to the weight of the surrounding air in the volume occupied by the balloon and the weight. We have highlighted the word "surrounding" in italics, because the density of atmospheric air changes during ascent for two reasons: firstly, its pressure decreases, and secondly, its temperature decreases.
We denote the volume of the ball as V. The volume of the load and the shell of the ball is usually neglected compared to the volume of the ball itself, but the masses of the load and the shell of the ball are of great importance! We denote the mass of the cargo m gr, and the mass of the shell - m vol. Then
F t.sh \u003d (m int + m about) g,
where m ext is the mass of gas with which the ball is filled.
Let us denote the density of the air surrounding the ball as ρ ext, and the density of the gas inside the ball as ρ ext.
21. Explain why the following equations are true:
F A = ρ ext gV,
m int = ρ int V,
V(ρ ext - ρ ext) = m gr + m vol. (4)
Clue. Use equation (3) and the relationship between mass, volume and density.
The underground force of a balloon is the weight of the load that this balloon can lift.
22. Explain why the modulus of the lift force of a balloon is expressed by the formula
F sub \u003d Vg (ρ external - ρ internal) - m about g. (5)
From formulas (4) and (5) it follows that the balloon can lift the load only if the density of the gas with which the balloon is filled is less than the density of the surrounding air.
If the balloon were rigid, this could be achieved by partially deflating the air from it: a rigid shell would be able to withstand the difference in air pressure inside and outside the balloon. However, the shell of a rigid ball would be too heavy. The soft shell, which is always used for balloons, cannot withstand any significant pressure difference. Therefore, the pressure of the gas inside the sphere is equal to the pressure of the surrounding air.
23. Explain why if the pressure inside the ball is equal to the pressure of the surrounding air, then the equality
ρ int / ρ ext = (M int * T ext) / (M ext * T int). (6)
Clue. Use the ideal gas equation of state.
It can be seen from formula (6) that the density of the gas with which the balloon is filled can be made less than the density of the surrounding air in two ways:
- use heated air as "internal" gas;
– use a gas with a lower molar mass.
The first method is used for pleasure balloons (Fig. 46.6), and the second - for meteorological probes (Fig. 46.7), which rise to a great height (in this case, the balloon is usually filled with helium). 
24. Explain why it follows from formulas (5) and (6) that the balloon lift modulus is expressed by the formula

? 25. A balloon with a volume of 3000 m 3 has an opening in the lower part through which the air inside the balloon is heated by a burner to a temperature of 77 ºС. The ball is in equilibrium at a height where the ambient temperature is 7 ºС and its density is 1.2 kg/m 3 . The mass of the shell of the ball is 300 kg. What is the weight of the load?
Additional questions and tasks
26. Air is pumped into the pontoon lying on the bottom of the lake at a depth of 90 m (Fig. 46.8). With an atom, water is forced out of the pontoon through a hole located in its lower part. What volume of atmospheric air must be supplied to the pontoon so that it can lift the load, if the total mass of the pontoon with the load is 20 tons, and the total volume of the load and the walls of the pontoon is 5 m 3? Assume that the water temperature is close to 0 ºС and the atmospheric pressure is 10 5 Pa. 
27. In the sealed elbow of a U-shaped tube there is a column of air 30 cm high. Mercury in both knees is at the same level. What will be the height of the air column if mercury is slowly added to the top? The pressure is equal to normal atmospheric pressure.
28. A balloon filled with helium is in equilibrium in the air. The mass of one square meter of the balloon shell is 50 g, the temperature of air and helium is 27 ºС, the pressure is equal to normal atmospheric pressure. What is the radius of the sphere?
Terms denoting the flow rate of the consumed respiratory mixture:
RMV - respiratory minute volume - breath volume per minute;
SAC - surface air consumption - surface air consumption.
Why does each diver need to know his own consumption of breathing mixture (air, nitrox, trimix - hereinafter for simplicity - gas)? The answer to this question is very simple. In order to correctly plan your dive and avoid a situation where gas suddenly ran out during a dive. What is needed for this? The process of measuring gas consumption is very simple, but requires several conditions to be met during the dive. First you need to understand that the gas consumption in different sailing conditions (depth, currents, speed, etc.) will be different. The greater the physical load on the body, the more CO2 is released and we breathe more. Therefore, you need to make several measurements:
- low load (drift with the flow, slow movement);
- average load (swimming without current at an average pace);
- a large load (swimming against the current or a fast pace of swimming).
In all these cases, we need to measure our gas intake. We dive to a pre-planned depth and, trying to adhere to it as strictly as possible, we make records of the following indicators - time, pressure in the cylinder, depth. For measurement accuracy, it is desirable to use half of the total gas supply for each measurement. Those. all three measurements can be made in 3 dives. During the entire measurement time, we must record the readings of the instruments with an interval of 3-10 minutes (depending on the diving conditions). As a result, you will get a table like this:
| T | P | D |
| 3 | 190 | 15,3 |
| 8 | 170 | 15,7 |
| 13 | 150 | 15,1 |
| 18 | 130 | 14,9 |
| 23 | 110 | 15,2 |
| 28 | 90 | 15 |
- T - current dive time, minutes,
- P is the pressure in the cylinder, bar,
- D - current depth, meters.
Next, we must calculate how much air, expressed in bars, we consume per minute. T total = (28-3) = 25 minutes P total = (190-90) = 100 bar 100/25 = 4 bar/min Next, we need to convert this value to liters. Knowing the volume of our cylinder (for example, for measurement we dived with a 12l steel cylinder) and the number of bars used, we can get a value in liters. 4*12 = 48 litres/min But we took our measurements under water, so we inhaled air at ambient pressure. We need surface flow for our planning. We calculate the average depth of immersion. Daverage = (15.3+15.7+15.1+14.9+15.2+15.0)/6 = 15.2 m P = (15.2/10)+1 = 2.52 ata Dividing our consumption by depth by the absolute pressure at that depth, we get the surface air flow in liters. RMV = 48 / 2.52 = 19.04 liters.

Having made three measurements in different conditions, we will have three different values that can be successfully used for further planning of our dives. Knowing the conditions in which we will dive and the depth of our dive, it will not be difficult for us to calculate how long this or that gas supply will last for us. Knowing this, we can better plan the dive itself and avoid disappointment from unreached goals. For example, we have an air tank with a volume of 12 liters and a pressure of 180 bar. The total volume of air in liters is 180*12 = 2160 liters. But for planning, we must immediately discard the "untouchable" gas supply of 28-35 bar, which we may need in emergency situations. So (180-35)*12 = 1740 liters of air per dive. The depth of the proposed dive is 25 meters. The dive will take place in a place without currents. The point of entry and exit to the water are in the same place. It is logical that in order to make such a dive, we will spend half of our free gas supply to the turning point, and the second on the way back. The pressure of the consumed air will be 25/10+1 = 3.5 atm then: 1740/2 = 870 liters. 870 / (19.04 * 3.5) = 12.81 min = 12 min (all rounding down to increase safety) Therefore, we have 12 minutes to travel to the dive target and inspect it and 12 minutes to return . When calculating multi-level dives, it is also possible to accurately calculate our consumption by dividing the dive into separate segments by depth and time.