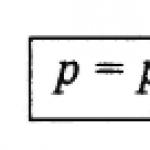Gas mixtures. Dalton's law
Partial pressure is that part of the total pressure of a gas mixture that is due to a given gas or steam. The partial gas in the mixture is equal to the pressure of the gas in the mixture that it would have alone, occupying the same volume as the mixture occupies at the same temperature.
Dalton's law.In the absence of chemical reactions, the total pressure of the gas mixture P total is equal to the sum of the partial pressures of all gases included in it p 1, p 2, p 3 ..., p n ˸
P total = p 1 + p 2 + ... + p n. (62)
The partial pressure of a given gas is proportional to the fraction ᴇᴦο of molecules from the total number of molecules of the mixture (mole fraction)˸
p i = P total ·X i = P total · . (63)
Mole fraction X i is the ratio of the number of moles of a given substance - n i (or a certain type of particle) to the total number of moles of the substance (or particles) located in the system n i.
The mole fraction can be attributed either to the entire system or to some phase. In the latter case, the ratio of the number of moles of a given substance in this phase to the total number of moles of the substance forming a given phase is taken. The sum of the mole fractions of all substances forming a system (or phase) is equal to unity.
The composition of gas mixtures can also be expressed using weight and volumetric parts. The weight fraction of a given gas in a mixture is the ratio of the mass of this gas to the mass of the gas mixture. If we denote the weight fractions of gases by G 1, G 2, G 3, ..., G i; and the masses of gases in the mixture - through m 1, m 2, m 3, ..., m i and the total mass of the gas mixture - through m, then we get˸
G 1 = G 2 = G 3 = … G n = (64)
G 1 + G 2 + G 3 + … + G n =1
m 1 + m 2 + m 3 + … + m n = m.
To express the composition of a gas mixture in volumetric units, it is necessary to bring the volumes of the gases that make up the mixture to the same pressure and temperature. The volume of an individual gas included in a mixture reduced to the pressure of the mixture is called reduced volume. In order to find the reduced volume of gas at the pressure of the gas mixture Ptot and temperature T, it is necessary to use the Boyle-Mariotte law˸
p 1 V total = v 1 P total; p 2 V total = v 2 P total; p 3 V total = v 3 P total; ... ; p n V total = v n P total,
where v 1, v 2, v 3, ..., v n are the reduced volumes of the individual gases that make up the mixture; р 1, р 2, р 3, …, р n – partial pressures of individual gases;
v 1 = v 2 = v 3 = …; v n = (65)
The sum of the reduced volumes of individual gases equals the total volume of the mixture˸
v 1 + v 2 + v 3 + … + v n = V total.
The ratio of the reduced volumes of individual gases to the total volume of the mixture is called the volume fraction and is expressed through r˸
r 1 = r 2 = r 3 = ...; r n = (66)
For gas mixtures, the composition expressed by volume and mole fractions is the same, i.e.˸
Gas mixtures. Dalton's law - concept and types. Classification and features of the category "Gas mixtures. Dalton's Law" 2015, 2017-2018.
It is worth mentioning two more gas laws. One of them concerns the number of molecules of different gases at the same pressures and temperatures, and the other concerns the mixture of gases.
Avogadro's law
At the beginning of the 19th century. the rule of multiple ratios was established for gases that enter into a chemical reaction. If the temperatures and pressures of gases combining with each other are equal, then their volumes are in simple ratios: 1:1, 1:2, 1:3, etc. Based on this rule, Avogadro in 1811 expressed a bold statement for this time hypothesis: Equal volumes of gases at the same temperatures and pressures contain the same number of molecules. At a ratio of 1:1, the molecules of the reacting gases are connected in pairs. If the volume ratio is 1:2, then each molecule of the first gas attaches to itself two molecules of the second, etc.
Currently, Avogadro's hypothesis has been strictly proven and is called Avogadro's law.
According to Avogadro's law different gases, taken in an amount of 1 mole, have the same volumes at the same pressuresR and temperaturest , since the number of molecules in them is the same. Under normal conditions, i.e. at a temperature of 0 °C and an atmospheric pressure of 101,325 Pa, this volume, as measurements show, is equal to
Volume V M 0 called molar.
Why is it that in equal volumes of gases at the same pressures and temperatures the same number of molecules is always found, regardless of which gas is taken? This can only be explained using molecular kinetic energy (see §4.5).
Dalton's law
More often they deal not with pure gas - oxygen, hydrogen, etc., but with a mixture of gases. Atmospheric air, in particular, is a mixture of nitrogen, oxygen and many other gases. Each of the gases in the mixture makes its own “contribution” to the total pressure on the walls of the vessel. The pressure that each of the gases making up the mixture would have if the remaining gases were removed from the vessel is called partial (i.e. private) pressure.
The simplest assumption that can be made is that the pressure of the gas mixture R equal to the sum of the partial pressures of all gases p 1 , R 2 , R 3 ...:
 (3.8.2)
(3.8.2)
The English chemist D. Dalton found that for sufficiently rarefied gases this is exactly the case in reality. Relationship (3.8.2) is called Dalton's law.
From the point of view of molecular kinetic theory, Dalton's law is satisfied because the interaction between the molecules of an ideal gas is negligible. Therefore, each gas exerts the same pressure on the wall of the vessel as if there were no other gases.
A mole of any gas under normal conditions occupies a volume 22.4 l. This volume value was established experimentally. In a mixture of gases, each of them exerts pressure on the walls of the container, regardless of the presence of other gases.
§ 3.9. Ideal gas equation of state
The state of a given mass of gas is characterized by three macroscopic parameters: pressure p, volumeVand temperature T. Now we will find the connection between them.
Equation of state
In § 3.5 and 3.6 you became familiar with the behavior of an ideal gas under specially created conditions. Two parameters out of three (p, V or V, T) changed at a constant value of the third (G or R). Typically, in nature and technology, gas changes all three parameters at once. For example, when air heated at the surface of the Earth rises, it expands, its pressure decreases and the temperature decreases.
Using gas laws (3.5.2) and (3.7.8), we can obtain an equation relating all three parameters p, V And T, characterizing the state of a gas of a given mass. This equation is called the ideal gas equation of state.
If a gas consists of a mixture of several gases, then Dalton’s law will help to calculate the pressure of the mixture
Where p v p 2 , ръ - partial pressures gases included in the mixture.
Partial pressure is the pressure that a gas would have if it alone occupied the entire available volume.
Molecular kinetic theory(MKT) arose in the 19th century. and presented the structure of matter (mainly gases) from the point of view of three provisions:
- all bodies consist of particles: atoms and molecules;
- particles are in continuous chaotic motion (thermal);
- particles interact with each other through perfectly elastic collisions.
MCT has become one of the most successful physical theories and has been confirmed by a number of experimental facts. A clear experimental confirmation of the chaotic thermal motion of atoms and molecules was Brownian motion.
Brownian motion - this phenomenon was discovered by Robert Brown 1 in 1827. Observing through a microscope the movement of flower pollen suspended in water, he saw disordered zigzag trajectories of particles.
The cause of Brownian motion is the thermal motion of the molecules of the medium, which is caused by pressure fluctuations. The impacts of the molecules of the medium lead the particle into random motion: its speed quickly changes in magnitude and direction. A complete theory of Brownian motion was given later by Albert Einstein and Marian Smoluchowski.
Basic MKT equation. The pressure of a gas on the wall of a vessel is determined by the impulse that gas molecules impart to the wall of the vessel when they collide with it. The higher the speed of the molecule, the greater the impulse it carries, the stronger it acts on the wall, i.e. R ~ v. In addition, the greater the mass of the molecule T, the higher the impulse, R ~ T. The higher the concentration of molecules P, the more often collisions occur, therefore, R ~ P. Assuming that the pressure is distributed equally in all directions in space (x, z/, z), we finally write

Kinetic energy of one molecule E = mv / 2. Connecting the last two equations with each other, we get

The last equation is called basic equation of MKT. This equation indicates that the average kinetic energy of ideal gas molecules (E) proportional to its temperature T. Note that the equation is written for a monatomic ideal gas. For a polyatomic gas it will take the form

Where i- the number of degrees of freedom of a molecule already known to you. From equality

follows that root mean square speed molecules of a monatomic gas is equal to

The Maxwell distribution 1 is a probability distribution, often found in equal parts of physics (and not only), and underlies MCT. The Maxwell distribution is also applicable to electronic transfer processes, to describe the properties of individual molecules in a gas. Typically this distribution refers to the distribution of energies of molecules in a gas, but it can also be applied to the distribution of velocities, momenta, and modulus of molecules. It can also be expressed as a discrete distribution over many discrete energy levels or as a continuous distribution over some continuum of energy.
We will limit ourselves to considering only one application of the Maxwell distribution - the velocity distribution of gas molecules.
Mathematically, the Maxwell distribution function (Fig. 4.1) is written as follows:


Rice. 4.1.
Let us explain the mathematical meaning of the distribution function. Any distribution function (including Maxwell’s) shows the probability that a certain quantity (in our case, the speed of gas molecules v) takes on a certain specified value. Maxwell velocity distribution function f(v) shows the probability that the speed of a gas molecule is v.
In Fig. 4.1 on the speed distribution curve, three characteristic points are marked: o - most likely speed of the molecule (it corresponds to the maximum, since it has the highest probability, hence the name), r> sr - average speed molecules (its probability is slightly less) and g; kv - mean square speed (with even less probability).
Let's define mathematical expressions for all three speeds. To find the most probable speed that corresponds to the maximum value /( v), need to calculate df/dv, set it equal to zero and solve for v

James Clerk Maxwell (1831 - 1879) - British physicist and mathematician. He laid the foundations of modern classical electrodynamics (Maxwell's equations), introduced the concepts of displacement current and electromagnetic field into physics, predicted the existence of electromagnetic waves, the electromagnetic nature of light, is one of the founders of the kinetic theory of gases and the author of the principle of color photography.
Formulation of laws
Law on the total pressure of a mixture of gases
Law on the solubility of gas mixture components
At a constant temperature, the solubility in a given liquid of each of the components of the gas mixture located above the liquid is proportional to their partial pressure.
Limits of applicability
Both Dalton's laws are strictly satisfied for ideal gases. For real gases, these laws are applicable provided that their solubility is low and their behavior is close to that of an ideal gas.
History of discovery
The law of addition of partial pressures was formulated in 1801. At the same time, the correct theoretical justification, based on molecular kinetic theory, was made much later.
Notes
Wikimedia Foundation.
2010.
See what "Dalton's Laws" are in other dictionaries: DALTON'S LAWS - (Dalton Dalton): the first law, the total pressure of a mixture of ideal gases that do not chemically interact with each other is equal to the sum of the partial (see) individual gases that make up the mixture, i.e., the pressures that each gas would produce in ... ...
Big Polytechnic Encyclopedia Dalton's laws Concepts of modern natural science. Glossary of basic terms
The basic laws of chemistry can be divided into qualitative and quantitative. Contents 1 Qualitative laws 1.1 I. Gibbs’ phase law ... Wikipedia
DALTON'S LAWS- (more correctly Dalton, Dalton). 1. The law of multiple ratios, discovered by D., is that elements are included in a chemical. connections in ratios that are always multiples of certain prime numbers. So, if they have water, then for one part by weight of hydrogen... ... Great Medical Encyclopedia
DALTON'S LAWS: 1) the pressure of a mixture of gases that do not chemically interact with each other is equal to the sum of their partial pressures; 2) the solubility of a component of a gas mixture in a given liquid at a constant temperature is proportional to the partial... ... Big Encyclopedic Dictionary
1) the pressure of a mixture of chemically non-interacting ideal gases is equal to the sum of the partial pressures. Approximately applicable to real gases at temperatures p and pressures far from critical. 2) At constant rate of solubility in a given liquid... ... Physical encyclopedia
1) the pressure of a mixture of chemically non-interacting ideal gases is equal to the sum of the partial pressures. Approximately applicable to real gases at temperatures and pressures far from critical. 2) At constant temperature solubility in a given ... Physical encyclopedia
DALTON'S LAWS: 1) the pressure of a mixture of gases that do not chemically interact with each other is equal to the sum of their partial pressures; 2) the solubility of a component of a gas mixture in a given liquid at a constant temperature is proportional to the partial... ... encyclopedic Dictionary
Describe processes occurring in equilibrium “liquid solution-vapor” systems under the influence of temperature or pressure. Contents 1 Konovalov’s first law 2 Konovalov’s second law ... Wikipedia
This article or section needs revision. Please improve the article in accordance with the rules for writing articles. The whole ... Wikipedia
Gas mixtures in which the components do not interact with each other can be described using Dalton's law. It relates the partial pressures of the components and their mole fractions into one equality. Let's take a closer look at this law, and also show how it can be used with specific examples.
Ideal gases
Dalton's law in physics turns out to be valid exclusively for ideal gases. These are gases whose constituent particles (atoms, molecules) do not interact with each other. For an ideal gas with a constant number of molecules (atoms) in it (n = const), an equality is valid that relates three macroscopic parameters (pressure P, volume V and temperature T):
P*V = n*R*T, R = 8.314 J/(K*mol) is a constant value.
All real gases at pressures of several atmospheres and temperatures of the order of room temperature and above can be considered ideal with good accuracy, that is, the above equality is valid for them.
Component Partial Pressure
To understand the essence of Dalton's law, it is necessary to understand the concept of "partial pressure".
Since molecules of different gases do not “feel” each other, for each chemical component i in the gas mixture the equality will be true:
The pressure Pi is called partial for the i-th component. In other words, partial pressure is the pressure that only the i-th component creates on the walls of the vessel. It is called partial because it is part of the total pressure, or its portion.
Statement of Dalton's law

In the early years of the 19th century, while studying the behavior of various gas mixtures, the British scientist John Dalton established the following fact: if you sum up all the partial pressures of the components of a gas mixture, you get a total pressure that can be measured with a barometer, pressure gauge or other device designed for this. This is Dalton's law. Let's write it in the form of a mathematical equality:
You can understand why this equality is true if you remember that the components of the mixture create pressure independently of each other.
Considering that the partial pressure Pi is directly proportional to the amount of substance ni component i, which is always true when T = const and V = const, then we come to another equality:
The quantity xi is called the mole fraction. It is related to the atomic percentages ai of the component by a simple relation:
An expression that allows one to determine the mole fraction of a component through its partial pressure and vice versa is also called Dalton's law.

It should not be forgotten that the law considered is valid not only in the case of ideal gases, but also in the absence of chemical reactions in them. The latter lead to changes in the component and molar composition, which violates the law for the pressure of the gas mixture.
Examples of problem solving
In this paragraph, we will consider examples of the application of Dalton's law to solve practical problems.
Task 1. It is necessary to determine the partial pressure of the three main components in dry air.
From the literature, you can find out that since air is dry, its main components will be nitrogen (about 78%), oxygen (about 21%) and the noble gas argon (about 1%). Considering that the total air pressure at sea level is 1 atmosphere, and converting atomic percentages into mole fractions, we obtain the partial pressures for each component:
Pi = Ptot*xiPN2 = 1 *0.78 = 0.78 atm.PO2 = 1*0.21 = 0.21 atm.PAr = 1*0.01 = 0.01 atm.
Problem 2. There are two cylinders with clean gases. The first cylinder contains nitrogen with a temperature of 300 K, a volume of 10 liters and a pressure of 2 atmospheres. The second cylinder contains oxygen with a temperature of 300 K, but having a volume of 15 liters and a pressure of 1.5 atmospheres. Both cylinders were connected to each other. It is necessary to calculate the partial pressure of each component in the resulting mixture.

Let's start solving this problem by calculating the amount of substance for nitrogen and oxygen. Using the ideal gas equation, we obtain:
PN2*VN2 = nN2*R*T =>nN2 = PN2*VN2/R*T = 2*101325*10-2/(8.314*300) = 0.812 mol; nO2 = PO2*VO2/R*T = 1, 5*101325*1.5*10-2/(8.314*300) = 0.914 mol.
When two cylinders are connected, the gases are mixed so that each component takes up the entire volume of the two cylinders. The total pressure that will be in the system can be calculated using also the equation of state of an ideal gas:
Vtot = VN2+VO2 = 2.5*10-2 m3; n = nN2+nO2 = 0.812+0.914 = 1.726 mol. Ptot = n*R*T/Vtot = 1.726*8.314*300/(2.5*10 -2) = 172199.568 Pa or 1.7 atm.
Now you can apply the formulas of Dalton's law to calculate the partial pressures of oxygen and nitrogen:
PN2 = Ptot*nN2/n = 1.7*0.812/1.726 = 0.8 atm; PO2 = Ptot - PN2 = 1.7 - 0.8 = 0.9 atm.
The ratio of the resulting partial pressures of gases is equal to the ratio of the amounts of substance for them.