From inorganic substances of cells water makes up about 65% of its mass: in young fast-growing cells up to 95%, in old cells - about 60%. The role of water in cells is very large, it is a medium and a solvent, participates in most chemical reactions, the movement of substances, thermoregulation, the formation of cellular structures, and determines the volume and elasticity of the cell. Most substances enter and exit the body in an aqueous solution.
Organic matter- make up 20-30% of the cell composition. They can be simple(amino acids, glucose, fatty acids) and complex(proteins, polysaccharides, nucleic acids, lipids). The most important are proteins, fats, carbohydrates, and nucleic acids.
Proteins are the main and most complex substances of any cell. The size of a protein molecule is hundreds and thousands of times larger than the molecules of inorganic compounds. Protein molecules are formed from simple compounds - amino acids (natural proteins contain 20 amino acids). Combining in different sequences and quantities, they form a wide variety (up to 1000) of proteins. Their role in the life of a cell is enormous: the building material of the body, catalysts (enzyme proteins accelerate chemical reactions), transport (blood hemoglobin delivers oxygen and nutrients to cells and carries away carbon dioxide and decay products). Proteins perform a protective and energy function.
Carbohydrates are organic substances consisting of carbon, hydrogen and oxygen. The simplest of them are monosaccharides - hexose, fructose, glucose (found in fruits, honey), galactose (in milk) and polysaccharides - consisting of several simple carbohydrates. These include starch and glycogen. Carbohydrates are the main source of energy for all forms of cellular activity (movement, biosynthesis, secretion, etc.) and play the role of reserve substances.
Lipids are water-insoluble fats and fat-like substances. They are the main structural component of biological membranes. Lipids perform an energy function and contain fat-soluble vitamins.
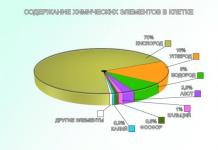
Nucleic acids - (from the Latin word “nucleus” - nucleus) - are formed in the nucleus of the cell. They come in two types: deoxyribonucleic acids (DNA) and ribonucleic acids (RNA). Their biological role is very great. They determine the synthesis of proteins and the transmission of hereditary information. Organisms are made up of cells. Cells of different organisms have similar chemical compositions. Table 1 presents the main chemical elements found in the cells of living organisms. Table 1. Content of chemical elements in the cell Based on the content in the cell, three groups of elements can be distinguished. The first group includes oxygen, carbon, hydrogen and nitrogen. They account for almost 98% of the total composition of the cell. The second group includes potassium, sodium, calcium, sulfur, phosphorus, magnesium, iron, chlorine. Their content in the cell is tenths and hundredths of a percent. Elements of these two groups are classified as macronutrients
(from Greek macro Table 1. Content of chemical elements in the cell - big). The remaining elements, represented in the cell by hundredths and thousandths of a percent, are included in the third group. This
No elements unique to living nature were found in the cell. All of the listed chemical elements are also part of inanimate nature. This indicates the unity of living and inanimate nature.
A deficiency of any element can lead to illness and even death of the body, since each element plays a specific role. Macroelements of the first group form the basis of biopolymers - proteins, carbohydrates, nucleic acids, as well as lipids, without which life is impossible. Sulfur is part of some proteins, phosphorus is part of nucleic acids, iron is part of hemoglobin, and magnesium is part of chlorophyll. Calcium plays an important role in metabolism.
Some of the chemical elements contained in the cell are part of inorganic substances - mineral salts and water.
Mineral salts are found in the cell, as a rule, in the form of cations (K +, Na +, Ca 2+, Mg 2+) and anions (HPO 2-/4, H 2 PO -/4, CI -, HCO 3), the ratio of which determines the acidity of the environment, which is important for the life of cells.
(In many cells, the environment is slightly alkaline and its pH almost does not change, since a certain ratio of cations and anions is constantly maintained in it.)
Of the inorganic substances in living nature, plays a huge role water.
Without water, life is impossible. It makes up a significant mass of most cells. A lot of water is contained in the cells of the brain and human embryos: more than 80% water; in adipose tissue cells - only 40.% By old age, the water content in cells decreases. A person who has lost 20% of water dies.
The unique properties of water determine its role in the body. It is involved in thermoregulation, which is due to the high heat capacity of water - the consumption of a large amount of energy when heating. What determines the high heat capacity of water?
In a water molecule, an oxygen atom is covalently bonded to two hydrogen atoms. The water molecule is polar because the oxygen atom has a partially negative charge, and each of the two hydrogen atoms has
Partially positive charge. A hydrogen bond is formed between the oxygen atom of one water molecule and the hydrogen atom of another molecule. Hydrogen bonds provide the connection of a large number of water molecules. When water is heated, a significant part of the energy is spent on breaking hydrogen bonds, which determines its high heat capacity.
Water - good solvent. Due to their polarity, its molecules interact with positively and negatively charged ions, thereby promoting the dissolution of the substance. In relation to water, all cell substances are divided into hydrophilic and hydrophobic.
Hydrophilic Table 1. Content of chemical elements in the cell hydro- water and filleo- love) are called substances that dissolve in water. These include ionic compounds (for example, salts) and some non-ionic compounds (for example, sugars).
Hydrophobic Table 1. Content of chemical elements in the cell hydro- water and Phobos- fear) are substances that are insoluble in water. These include, for example, lipids.
Water plays an important role in the chemical reactions that occur in the cell in aqueous solutions. It dissolves metabolic products that the body does not need and thereby promotes their removal from the body. The high water content in the cell gives it elasticity. Water facilitates the movement of various substances within a cell or from cell to cell.
Bodies of living and inanimate nature consist of the same chemical elements. Living organisms contain inorganic substances - water and mineral salts. The vitally important numerous functions of water in a cell are determined by the characteristics of its molecules: their polarity, the ability to form hydrogen bonds.
INORGANIC COMPONENTS OF THE CELL
About 90 elements are found in the cells of living organisms, and about 25 of them are found in almost all cells. Based on their content in the cell, chemical elements are divided into three large groups: macroelements (99%), microelements (1%), ultramicroelements (less than 0.001%).
Macroelements include oxygen, carbon, hydrogen, phosphorus, potassium, sulfur, chlorine, calcium, magnesium, sodium, iron.
Microelements include manganese, copper, zinc, iodine, fluorine.
Ultramicroelements include silver, gold, bromine, and selenium.
| ELEMENTS | CONTENT IN THE BODY (%) | BIOLOGICAL SIGNIFICANCE |
| Macronutrients: | ||
| O.C.H.N. | 62-3 | Contains all organic substances in cells, water |
| Phosphorus R | 1,0 | They are part of nucleic acids, ATP (forms high-energy bonds), enzymes, bone tissue and tooth enamel |
| Calcium Ca +2 | 2,5 | In plants it is part of the cell membrane, in animals - in the composition of bones and teeth, activates blood clotting |
| Microelements: | 1-0,01 | |
| Sulfur S | 0,25 | Contains proteins, vitamins and enzymes |
| Potassium K+ | 0,25 | Causes the conduction of nerve impulses; activator of protein synthesis enzymes, photosynthesis processes, plant growth |
| Chlorine CI - | 0,2 | It is a component of gastric juice in the form of hydrochloric acid, activates enzymes |
| Sodium Na+ | 0,1 | Ensures the conduction of nerve impulses, maintains osmotic pressure in the cell, stimulates the synthesis of hormones |
| Magnesium Mg +2 | 0,07 | Part of the chlorophyll molecule, found in bones and teeth, activates DNA synthesis and energy metabolism |
| Iodine I - | 0,1 | Part of the thyroid hormone - thyroxine, affects metabolism |
| Iron Fe+3 | 0,01 | It is part of hemoglobin, myoglobin, the lens and cornea of the eye, an enzyme activator, and is involved in the synthesis of chlorophyll. Provides oxygen transport to tissues and organs |
| Ultramicroelements: | less than 0.01, trace amounts | |
| Copper Si +2 | Participates in the processes of hematopoiesis, photosynthesis, catalyzes intracellular oxidative processes | |
| Manganese Mn | Increases plant productivity, activates the process of photosynthesis, affects hematopoietic processes | |
| Bor V | Affects plant growth processes | |
| Fluorine F | It is part of the enamel of teeth; if there is a deficiency, caries develops; if there is an excess, fluorosis develops. | |
| Substances: | ||
| N 2 0 | 60-98 | It makes up the internal environment of the body, participates in hydrolysis processes, and structures the cell. Universal solvent, catalyst, participant in chemical reactions |
ORGANIC COMPONENTS OF CELLS
| SUBSTANCES | STRUCTURE AND PROPERTIES | FUNCTIONS |
| Lipids | ||
| Esters of higher fatty acids and glycerol. The composition of phospholipids additionally includes the residue H 3 PO4. They have hydrophobic or hydrophilic-hydrophobic properties and high energy intensity | Construction- forms the bilipid layer of all membranes. Energy. Thermoregulatory. Protective. Hormonal(corticosteroids, sex hormones). Components of vitamins D, E. Source of water in the body. Reserve nutrient |
|
| Carbohydrates | ||
| Monosaccharides: glucose, fructose, ribose, deoxyribose |
Highly soluble in water | Energy |
| Disaccharides: sucrose, maltose (malt sugar) |
Soluble in water | Components DNA, RNA, ATP |
| Polysaccharides: starch, glycogen, cellulose |
Poorly soluble or insoluble in water | Spare nutrient. Construction - the shell of a plant cell |
| Squirrels | Polymers. Monomers - 20 amino acids. | Enzymes are biocatalysts. |
| I structure is the sequence of amino acids in the polypeptide chain. Bond - peptide - CO-NH- | Construction - are part of membrane structures, ribosomes. | |
| II structure - a-helix, bond - hydrogen | Motor (contractile muscle proteins). | |
| III structure - spatial configuration a-spirals (globule). Bonds - ionic, covalent, hydrophobic, hydrogen | Transport (hemoglobin). Protective (antibodies). Regulatory (hormones, insulin) | |
| The IV structure is not characteristic of all proteins. Connection of several polypeptide chains into a single superstructure. Poorly soluble in water. The action of high temperatures, concentrated acids and alkalis, heavy metal salts causes denaturation | ||
| Nucleic acids: | Biopolymers. Made up of nucleotides | |
| DNA is deoxyribonucleic acid. | Nucleotide composition: deoxyribose, nitrogenous bases - adenine, guanine, cytosine, thymine, H 3 PO 4 residue. Complementarity of nitrogenous bases A = T, G = C. Double helix. Capable of self-doubling | They form chromosomes. Storage and transmission of hereditary information, genetic code. Biosynthesis of RNA and proteins. Encodes the primary structure of a protein. Contained in the nucleus, mitochondria, plastids |
| RNA is ribonucleic acid. | Nucleotide composition: ribose, nitrogenous bases - adenine, guanine, cytosine, uracil, H 3 PO 4 residue Complementarity of nitrogenous bases A = U, G = C. One chain | |
| Messenger RNA | Transfer of information about the primary structure of the protein, participates in protein biosynthesis | |
| Ribosomal RNA | Builds the ribosome body | |
| Transfer RNA | Encodes and transports amino acids to the site of protein synthesis - ribosomes | |
| Viral RNA and DNA | Genetic apparatus of viruses | |
Enzymes.
The most important function of proteins is catalytic. Protein molecules that increase the rate of chemical reactions in a cell by several orders of magnitude are called enzymes. Not a single biochemical process in the body occurs without the participation of enzymes.
Currently, over 2000 enzymes have been discovered. Their efficiency is many times higher than the efficiency of inorganic catalysts used in production. Thus, 1 mg of iron in the catalase enzyme replaces 10 tons of inorganic iron. Catalase increases the rate of decomposition of hydrogen peroxide (H 2 O 2) by 10 11 times. The enzyme that catalyzes the reaction of carbonic acid formation (CO 2 + H 2 O = H 2 CO 3) accelerates the reaction 10 7 times.
An important property of enzymes is the specificity of their action; each enzyme catalyzes only one or a small group of similar reactions.
The substance that the enzyme acts on is called substrate. The structures of the enzyme and substrate molecules must exactly match each other. This explains the specificity of the action of enzymes. When a substrate is combined with an enzyme, the spatial structure of the enzyme changes.
The sequence of interaction between enzyme and substrate can be depicted schematically:
Substrate+Enzyme - Enzyme-substrate complex - Enzyme+Product.
The diagram shows that the substrate combines with the enzyme to form an enzyme-substrate complex. In this case, the substrate is transformed into a new substance - a product. At the final stage, the enzyme is released from the product and again interacts with another substrate molecule.
Enzymes function only at a certain temperature, concentration of substances, and acidity of the environment. Changing conditions leads to changes in the tertiary and quaternary structure of the protein molecule, and, consequently, to the suppression of enzyme activity. How does this happen? Only a certain part of the enzyme molecule, called active center. The active center contains from 3 to 12 amino acid residues and is formed as a result of bending of the polypeptide chain.
Under the influence of various factors, the structure of the enzyme molecule changes. In this case, the spatial configuration of the active center is disrupted, and the enzyme loses its activity.
Enzymes are proteins that act as biological catalysts. Thanks to enzymes, the rate of chemical reactions in cells increases by several orders of magnitude. An important property of enzymes is their specificity of action under certain conditions.
Nucleic acids.
Nucleic acids were discovered in the second half of the 19th century. Swiss biochemist F. Miescher, who isolated a substance with a high content of nitrogen and phosphorus from cell nuclei and called it “nuclein” (from lat. core- core).
Nucleic acids store hereditary information about the structure and functioning of every cell and all living beings on Earth. There are two types of nucleic acids - DNA (deoxyribonucleic acid) and RNA (ribonucleic acid). Nucleic acids, like proteins, have species specificity, that is, organisms of each species have their own type of DNA. To find out the reasons for species specificity, consider the structure of nucleic acids.
Nucleic acid molecules are very long chains consisting of many hundreds and even millions of nucleotides. Any nucleic acid contains only four types of nucleotides. The functions of nucleic acid molecules depend on their structure, the nucleotides they contain, their number in the chain and the sequence of the compound in the molecule.
Each nucleotide consists of three components: a nitrogenous base, a carbohydrate and a phosphoric acid. Each DNA nucleotide contains one of four types of nitrogenous bases (adenine - A, thymine - T, guanine - G or cytosine - C), as well as deoxyribose carbon and a phosphoric acid residue.
Thus, DNA nucleotides differ only in the type of nitrogenous base.
The DNA molecule consists of a huge number of nucleotides connected in a chain in a certain sequence. Each type of DNA molecule has its own number and sequence of nucleotides.
DNA molecules are very long. For example, to write down the sequence of nucleotides in DNA molecules from one human cell (46 chromosomes) in letters would require a book of about 820,000 pages. The alternation of four types of nucleotides can form an infinite number of variants of DNA molecules. These structural features of DNA molecules allow them to store a huge amount of information about all the characteristics of organisms.
In 1953, the American biologist J. Watson and the English physicist F. Crick created a model of the structure of the DNA molecule. Scientists have found that each DNA molecule consists of two chains, interconnected and spirally twisted. It looks like a double helix. In each chain, four types of nucleotides alternate in a specific sequence.
The nucleotide composition of DNA varies among different types of bacteria, fungi, plants, and animals. But it does not change with age and depends little on environmental changes. Nucleotides are paired, that is, the number of adenine nucleotides in any DNA molecule is equal to the number of thymidine nucleotides (A-T), and the number of cytosine nucleotides is equal to the number of guanine nucleotides (C-G). This is due to the fact that the connection of two chains to each other in a DNA molecule is subject to a certain rule, namely: adenine of one chain is always connected by two hydrogen bonds only with Thymine of the other chain, and guanine - by three hydrogen bonds with cytosine, that is, the nucleotide chains of one molecule DNA is complementary, complementing each other.
Nucleic acid molecules - DNA and RNA - are made up of nucleotides. DNA nucleotides include a nitrogenous base (A, T, G, C), the carbohydrate deoxyribose and a phosphoric acid molecule residue. The DNA molecule is a double helix, consisting of two chains connected by hydrogen bonds according to the principle of complementarity. The function of DNA is to store hereditary information.
The cells of all organisms contain molecules of ATP - adenosine triphosphoric acid. ATP is a universal cell substance, the molecule of which has energy-rich bonds. The ATP molecule is one unique nucleotide, which, like other nucleotides, consists of three components: a nitrogenous base - adenine, a carbohydrate - ribose, but instead of one it contains three residues of phosphoric acid molecules (Fig. 12). The connections indicated in the figure with an icon are rich in energy and are called macroergic. Each ATP molecule contains two high-energy bonds.
When a high-energy bond is broken and one molecule of phosphoric acid is removed with the help of enzymes, 40 kJ/mol of energy is released, and ATP is converted into ADP - adenosine diphosphoric acid. When another molecule of phosphoric acid is removed, another 40 kJ/mol is released; AMP is formed - adenosine monophosphoric acid. These reactions are reversible, that is, AMP can be converted into ADP, ADP into ATP.
ATP molecules are not only broken down, but also synthesized, so their content in the cell is relatively constant. The importance of ATP in the life of a cell is enormous. These molecules play a leading role in the energy metabolism necessary to ensure the life of the cell and the organism as a whole.
Rice. 12. Scheme of the structure of ATP.| adenine - |
An RNA molecule is usually a single chain, consisting of four types of nucleotides - A, U, G, C. Three main types of RNA are known: mRNA, rRNA, tRNA. The content of RNA molecules in a cell is not constant; they participate in protein biosynthesis. ATP is a universal energy substance of the cell, which contains energy-rich bonds. ATP plays a central role in cellular energy metabolism. RNA and ATP are found in both the nucleus and cytoplasm of the cell.
Tasks and tests on the topic "Topic 4. "Chemical composition of the cell."
- polymer, monomer;
- carbohydrate, monosaccharide, disaccharide, polysaccharide;
- lipid, fatty acid, glycerol;
- amino acid, peptide bond, protein;
- catalyst, enzyme, active site;
- nucleic acid, nucleotide.
Algorithm for solving problems.
Type 1. Self-copying of DNA.
One of the DNA chains has the following nucleotide sequence:
AGTACCGATACCGATTTACCG...
What nucleotide sequence does the second chain of the same molecule have?
To write the nucleotide sequence of the second strand of a DNA molecule, when the sequence of the first strand is known, it is enough to replace thymine with adenine, adenine with thymine, guanine with cytosine, and cytosine with guanine. Having made this replacement, we get the sequence:
TATTGGGCTATGAGCTAAAATG...
Type 2. Protein coding.
The chain of amino acids of the ribonuclease protein has the following beginning: lysine-glutamine-threonine-alanine-alanine-alanine-lysine...
What nucleotide sequence does the gene corresponding to this protein begin with?
To do this, use the genetic code table. For each amino acid, we find its code designation in the form of the corresponding triple of nucleotides and write it down. Arranging these triplets one after another in the same order as the corresponding amino acids, we obtain the formula for the structure of a section of messenger RNA. As a rule, there are several such triplets, the choice is made according to your decision (but only one of the triplets is taken). Accordingly, there may be several solutions.
ААААААААЦУГЦГГЦУГЦГАAG
What sequence of amino acids does a protein begin with if it is encoded by the following sequence of nucleotides:
ATCGCCATGGGGCCGGT...
Using the principle of complementarity, we find the structure of a section of messenger RNA formed on a given segment of a DNA molecule:
UGGGGGUATCCGGCCCA...
Then we turn to the table of the genetic code and for each triple of nucleotides, starting from the first, we find and write out the corresponding amino acid:
Cysteine-glycine-tyrosine-arginine-proline-...
Ivanova T.V., Kalinova G.S., Myagkova A.N. "General Biology". Moscow, "Enlightenment", 2000
- Topic 4. "Chemical composition of the cell." §2-§7 pp. 7-21
- Topic 5. "Photosynthesis." §16-17 pp. 44-48
- Topic 6. "Cellular respiration." §12-13 pp. 34-38
- Topic 7. "Genetic information." §14-15 pp. 39-44
Cell
From the point of view of the concept of living systems according to A. Lehninger.
A living cell is an isothermal system of organic molecules capable of self-regulation and self-reproduction, extracting energy and resources from the environment.
A large number of sequential reactions take place in a cell, the speed of which is regulated by the cell itself.
The cell maintains itself in a stationary dynamic state, far from equilibrium with the environment.
Cells function on the principle of minimal consumption of components and processes.
That. A cell is an elementary living open system capable of independent existence, reproduction and development. It is the elementary structural and functional unit of all living organisms.
Chemical composition of cells.
Of the 110 elements of Mendeleev’s periodic table, 86 were found to be constantly present in the human body.
25 of them are necessary for normal life, 18 of them are absolutely necessary, and 7 are useful. In accordance with the percentage content in the cell, chemical elements are divided into three groups:
Macroelements The main elements (organogens) are hydrogen, carbon, oxygen, nitrogen. Their concentration: 98 – 99.9%. They are universal components of organic cell compounds.
Microelements - sodium, magnesium, phosphorus, sulfur, chlorine, potassium, calcium, iron. Their concentration is 0.1%.
Ultramicroelements - boron, silicon, vanadium, manganese, cobalt, copper, zinc, molybdenum, selenium, iodine, bromine, fluorine. They affect metabolism. Their absence is the cause of diseases (zinc - diabetes mellitus, iodine - endemic goiter, iron - pernicious anemia, etc.).
Modern medicine knows facts about negative interactions between vitamins and minerals:
Zinc reduces copper absorption and competes with iron and calcium for absorption; (and zinc deficiency causes a weakening of the immune system and a number of pathological conditions on the part of the endocrine glands).
Calcium and iron reduce the absorption of manganese;
Vitamin E does not combine well with iron, and vitamin C does not combine well with B vitamins.
Positive interaction:
Vitamin D is necessary for the absorption of calcium;
Copper promotes the absorption and increases the efficiency of iron use in the body.
Inorganic components of the cell.
Water– the most important component of the cell, the universal dispersion medium of living matter. Active cells of terrestrial organisms consist of 60–95% water. In resting cells and tissues (seeds, spores) there is 10 - 20% water. Water in the cell is in two forms - free and bound to cellular colloids. Free water is the solvent and dispersion medium of the colloidal system of protoplasm. Its 95%. Bound water (4–5%) of all cell water forms weak hydrogen and hydroxyl bonds with proteins.
Properties of water:
Water is a natural solvent for mineral ions and other substances.
Water is the dispersive phase of the colloidal system of protoplasm.
Water is the medium for cell metabolic reactions, because physiological processes occur in an exclusively aquatic environment. Provides reactions of hydrolysis, hydration, swelling.
Participates in many enzymatic reactions of the cell and is formed during metabolism.
Water is a source of hydrogen ions during photosynthesis in plants.
Biological significance of water:
Most biochemical reactions occur only in aqueous solution; many substances enter and exit cells in dissolved form. This characterizes the transport function of water.
Water provides hydrolysis reactions - the breakdown of proteins, fats, carbohydrates under the influence of water.
Due to the high heat of evaporation, the body is cooled. For example, sweating in humans or transpiration in plants.
The high heat capacity and thermal conductivity of water contributes to the uniform distribution of heat in the cell.
Due to the forces of adhesion (water - soil) and cohesion (water - water), water has the property of capillarity.
The incompressibility of water determines the stressed state of cell walls (turgor) and the hydrostatic skeleton in roundworms.
All organisms on our planet consist of cells that are similar in chemical composition. In this article we will briefly talk about the chemical composition of the cell, its role in the life of the entire organism, and find out what science studies this issue.
Groups of elements of the chemical composition of the cell
The science that studies the components and structure of a living cell is called cytology.
All elements included in the chemical structure of the body can be divided into three groups:
- macroelements;
- microelements;
- ultramicroelements.
Macroelements include hydrogen, carbon, oxygen and nitrogen. They account for almost 98% of all constituent elements.
Microelements are present in tenths and hundredths of a percent. And a very low content of ultramicroelements - hundredths and thousandths of a percent.
TOP 4 articleswho are reading along with this
Translated from Greek, “macro” means big, and “micro” means small.

Scientists have found that there are no special elements that are unique to living organisms. Therefore, both living and inanimate nature consists of the same elements. This proves their relationship.
Despite the quantitative content of a chemical element, the absence or reduction of at least one of them leads to the death of the entire organism. After all, each of them has its own meaning.
The role of the chemical composition of the cell
Macroelements are the basis of biopolymers, namely proteins, carbohydrates, nucleic acids and lipids.
Microelements are part of vital organic substances and participate in metabolic processes. They are constituent components of mineral salts, which are in the form of cations and anions, their ratio determines the alkaline environment. Most often it is slightly alkaline, because the ratio of mineral salts does not change.
Hemoglobin contains iron, chlorophyll - magnesium, proteins - sulfur, nucleic acids - phosphorus, metabolism occurs with a sufficient amount of calcium.

Rice. 2. Cell composition
Some chemical elements are components of inorganic substances, such as water. It plays an important role in the life of both plant and animal cells. Water is a good solvent, because of this all substances inside the body are divided into:
- Hydrophilic - dissolves in water;
- Hydrophobic - do not dissolve in water.
Thanks to the presence of water, the cell becomes elastic, it promotes the movement of organic substances in the cytoplasm.

Rice. 3. Cell substances.
Table “Properties of the chemical composition of the cell”
To clearly understand what chemical elements are part of the cell, we included them in the following table:
|
Elements |
Meaning |
|
|
Macronutrients |
||
|
Oxygen, carbon, hydrogen, nitrogen |
||
|
A constituent component of the shell in plants, in animals it is found in bones and teeth, and takes an active part in blood clotting. |
||
|
Contained in nucleic acids, enzymes, bone tissue and tooth enamel. |
||
|
Microelements |
||
|
It is the basis of proteins, enzymes and vitamins. |
||
|
Provides transmission of nerve impulses, activates protein synthesis, photosynthesis and growth processes. |
||
|
One of the components of gastric juice, an enzyme provocateur. |
||
|
Takes an active part in metabolic processes, a component of the thyroid hormone. |
||
|
Ensures the transmission of impulses in the nervous system, maintains constant pressure inside the cell, and provokes the synthesis of hormones. |
||
|
A constituent element of chlorophyll, bone tissue and teeth, provokes DNA synthesis and heat transfer processes. |
||
|
An integral part of hemoglobin, the lens, and the cornea, it synthesizes chlorophyll. Transports oxygen throughout the body. |
||
|
Ultramicroelements |
||
|
An integral part of the processes of blood formation and photosynthesis, it accelerates intracellular oxidation processes. |
||
|
Manganese |
Activates photosynthesis, participates in blood formation, and ensures high productivity. |
|
|
Component of tooth enamel. |
||
|
Regulates plant growth. |
||
What have we learned?
Each cell of living nature has its own set of chemical elements. In terms of their composition, objects of living and inanimate nature have similarities, this proves their close relationship. Each cell consists of macroelements, microelements and ultramicroelements, each of which has its own role. The absence of at least one of them leads to illness and even death of the entire organism.
Test on the topic
Evaluation of the report
Average rating: 4.5. Total ratings received: 922.
A cell is an elementary unit of a living thing, possessing all the characteristics of an organism: the ability to reproduce, grow, exchange substances and energy with the environment, irritability, and the constancy of chemical output.
Macroelements are elements whose amount in a cell is up to 0.001% of body weight. Examples are oxygen, carbon, nitrogen, phosphorus, hydrogen, sulfur, iron, sodium, calcium, etc.
Microelements are elements whose amount in a cell ranges from 0.001% to 0.000001% of body weight. Examples are boron, copper, cobalt, zinc, iodine, etc.
Ultramicroelements are elements whose content in a cell does not exceed 0.000001% of body weight. Examples are gold, mercury, cesium, selenium, etc.
2. Make a diagram of “Cell Substances”.
3. What does the scientific fact of the similarity of the elementary chemical composition of living and inanimate nature indicate?
This indicates the commonality of living and inanimate nature.
Inorganic substances. The role of water and minerals in cell life.
1. Give definitions of concepts.
Inorganic substances are water, mineral salts, acids, anions and cations present in both living and non-living organisms.
Water is one of the most common inorganic substances in nature, the molecule of which consists of two hydrogen atoms and one oxygen atom.
2. Draw a diagram of the “Structure of Water”.

3. What structural features of water molecules give it unique properties, without which life is impossible?
The structure of the water molecule is formed by two hydrogen atoms and one oxygen atom, which form a dipole, that is, water has two polarities “+” and “-”. This contributes to its permeability through the membrane walls, the ability to dissolve chemicals. In addition, water dipoles are connected by hydrogen bonds to each other, which ensures its ability to be in different states of aggregation, as well as to dissolve or not dissolve various substances.
4. Fill out the table “The role of water and minerals in the cell.”

5. What is the significance of the relative constancy of the internal environment of a cell in ensuring its vital processes?
The constancy of the internal environment of the cell is called homeostasis. Violation of homeostasis leads to damage to the cell or to its death, plastic metabolism and energy exchange are constantly occurring in the cell, these are two components of metabolism, and disruption of this process leads to damage or death of the entire organism.
6. What is the purpose of buffer systems of living organisms and what is the principle of their functioning?
Buffer systems maintain a certain pH value (an indicator of acidity) of the environment in biological fluids. The principle of operation is that the pH of the medium depends on the concentration of protons in this medium (H+). The buffer system is capable of absorbing or donating protons depending on their entry into the environment from the outside or, conversely, removal from the environment, while the pH will not change. The presence of buffer systems is necessary in a living organism, since due to changes in environmental conditions, pH can vary greatly, and most enzymes work only at a certain pH value.
Examples of buffer systems:
carbonate-hydrocarbonate (mixture of Na2СО3 and NaHCO3)
phosphate (mixture of K2HPO4 and KH2PO4).
Organic substances. The role of carbohydrates, lipids and proteins in cell life.
1. Give definitions of concepts.
Organic substances are substances that necessarily contain carbon; they are part of living organisms and are formed only with their participation.
Proteins are high molecular weight organic substances consisting of alpha amino acids linked into a chain by a peptide bond.
Lipids are a large group of natural organic compounds, including fats and fat-like substances. The molecules of simple lipids consist of alcohol and fatty acids, complex ones - of alcohol, high-molecular fatty acids and other components.
Carbohydrates are organic substances containing carbonyl and several hydroxyl groups and are otherwise called sugars.
2. Fill in the table with the missing information “Structure and functions of organic substances of the cell.”

3. What is meant by protein denaturation?
Protein denaturation is the loss of a protein's natural structure.
Nucleic acids, ATP and other organic compounds of the cell.
1. Give definitions of concepts.
Nucleic acids are biopolymers consisting of monomers - nucleotides.
ATP is a compound consisting of the nitrogenous base adenine, the carbohydrate ribose and three phosphoric acid residues.
A nucleotide is a nucleic acid monomer that consists of a phosphate group, a five-carbon sugar (pentose) and a nitrogenous base.
A macroergic bond is a bond between phosphoric acid residues in ATP.
Complementarity is the spatial mutual correspondence of nucleotides.
2. Prove that nucleic acids are biopolymers.
Nucleic acids consist of a large number of repeating nucleotides and have a mass of 10,000 to several million carbon units.
3. Describe the structural features of the nucleotide molecule.
A nucleotide is a compound of three components: a phosphoric acid residue, a five-carbon sugar (ribose), and one of the nitrogenous compounds (adenine, guanine, cytosine, thymine or uracil).
4. What is the structure of a DNA molecule?
DNA is a double helix consisting of many nucleotides that are sequentially connected to each other due to covalent bonds between the deoxyribose of one and the phosphoric acid residue of another nucleotide. The nitrogenous bases, which are located on one side of the backbone of one chain, are connected by H-bonds to the nitrogenous bases of the second chain according to the principle of complementarity.
5. Applying the principle of complementarity, construct the second strand of DNA.
T-A-T-C-A-G-A-C-C-T-A-C
A-T-A-G-T-C-T-G-G-A-T-G.
6. What are the main functions of DNA in a cell?
With the help of four types of nucleotides, DNA records all the important information in the cell about the organism, which is passed on to subsequent generations.
7. How does an RNA molecule differ from a DNA molecule?
RNA is a single strand smaller than DNA. Nucleotides contain the sugar ribose, not deoxyribose, as in DNA. The nitrogenous base, instead of thymine, is uracil.
8. What do the structures of DNA and RNA molecules have in common?
Both RNA and DNA are biopolymers made up of nucleotides. What nucleotides have in common in structure is the presence of a phosphoric acid residue and the bases adenine, guanine, and cytosine.
9. Complete the table “Types of RNA and their functions in the cell.”

10. What is ATP? What is its role in the cell?
ATP – adenosine triphosphate, a high-energy compound. Its functions are the universal storer and carrier of energy in the cell.
11. What is the structure of the ATP molecule?
ATP consists of three phosphoric acid residues, ribose and adenine.
12. What are vitamins? What two large groups are they divided into?
Vitamins are biologically active organic compounds that play an important role in metabolic processes. They are divided into water-soluble (C, B1, B2, etc.) and fat-soluble (A, E, etc.).
13. Fill out the table “Vitamins and their role in the human body.”