Compared to other groups of protozoa, ciliates have the most complex structure, which is associated with the diversity and complexity of their functions.
Where does the name “slipper ciliate” come from? You will not be surprised if you look under a microscope at a living ciliate or even at its image (Fig. 85).
Indeed, the body shape of this ciliate resembles an elegant lady's shoe.
The ciliate slipper is in continuous rather rapid movement. Its speed (at room temperature) is about 2.0-2.5 mm/sec. That's a lot of speed for such a small animal! After all, this means that in a second the shoe covers a distance exceeding the length of its body by 10-15 times. The trajectory of the shoe's movement is quite complex.
She moves her front end straight ahead
Ciliate shoe (PARAMECIUM CAUDATUM)
To become familiar with the structure and lifestyle of these interesting single-celled organisms, let us first turn to one typical example. Let's take the slipper ciliates (species of the genus Paramecium) that are widespread in small freshwater bodies of water. These ciliates are very easy to breed in small aquariums if you add pond water to ordinary meadow hay. In such tinctures, many different types of protozoa develop and almost always slipper ciliates develop. Using a regular educational microscope, you can examine much of what will be discussed below. Among the simplest ciliates slippers
are quite large organisms. Their body length is about 1/6-1/3 mm. and at the same time rotates to the right along the longitudinal axis of the body.
Such active movement of the shoe depends on the work of a large number of the finest hair-like appendages - cilia, which cover the entire body of the ciliate. The number of cilia in one individual slipper ciliate is 10-15 thousand!
When the shoe floats, the movements of the numerous cilia covering its body are summed up. The actions of individual cilia are coordinated, resulting in regular wave-like vibrations of all cilia. The vibration wave begins at the front end of the body and spreads backward. At the same time, 2-3 waves of contraction pass along the body of the shoe. Thus, the entire ciliary apparatus of the ciliate is, as it were, a single functional physiological whole, the actions of the individual structural units of which (cilia) are closely connected (coordinated) with each other.
The structure of each individual cilium of the shoe, as shown by electron microscopic studies, is very complex.
The direction and speed of movement of the shoe are not constant and unchanging quantities. The shoe, like all living organisms (we have already seen this in the example of amoeba), reacts to changes in the external environment by changing the direction of movement.
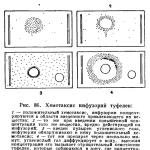
Changes in the direction of movement of protozoa under the influence of various stimuli are called taxis. It is easy to observe various taxis in ciliates. If you place some substance that has an unfavorable effect on them (for example, a crystal of table salt) in the drop where the shoes are floating, then the shoes float away (as if they run away) from this factor that is unfavorable for them (Fig. 86).

Here is an example of negative taxis to chemical influence (negative chemotaxis). Positive chemotaxis can also be observed in the slipper. If, for example, a drop of water in which ciliates are swimming is covered with a cover glass and a bubble of carbon dioxide (CO2) is placed under it, then most of the ciliates will head towards this bubble and arrange themselves in a ring around it.
The phenomenon of taxis is very clearly manifested in shoes under the influence of electric current. If a weak electric current is passed through the liquid in which the slippers are floating, then the following picture can be observed: all ciliates orient their longitudinal axis parallel to the current line, and then, as if on command, they move towards the cathode, in the area of which they form a dense cluster. The movement of ciliates, determined by the direction of the electric current, is called galvanotaxis. Various taxis in ciliates can be detected under the influence of a wide variety of environmental factors.
The entire cytoplasmic body of the ciliate is clearly divided into 2 layers: the outer one is lighter (ectoplasm) and the inner one is darker and granular (endoplasm). The most superficial layer of ectoplasm forms an outer very thin and at the same time durable and elastic shell - the pellicle, which plays an important role in maintaining the constancy of the body shape of the ciliates.
In the outer layer (ectoplasm) of the body of a living slipper, numerous short rods located perpendicular to the surface are clearly visible (Fig. 85, 7). These formations are called trichocysts. Their function is very interesting and is associated with the protection of protozoa. When mechanical, chemical or any other strong irritation occurs, the trichocysts are forcefully thrown out, turning into thin long threads that hit the predator attacking the shoe. Trichocysts represent a powerful defense. They are located regularly between the cilia, so that the number of trichocysts approximately corresponds to the number of cilia. In place of the used (“shot”) trichocysts, new ones develop in the ectoplasm of the slipper.
On one side, approximately in the middle of the body (Fig. 85, 5), the shoe has a rather deep depression. This is the oral cavity, or peristome. On the walls of the peristome, as well as on the surface of the body, cilia are located. They are developed here much more powerfully than on the rest of the body surface. These closely spaced cilia are collected in two groups. The function of these specially differentiated cilia is associated not with movement, but with nutrition (Fig. 87).

How and what do slippers eat, how do they digest?
Slippers are among the ciliates whose main food is bacteria. Along with bacteria, they can ingest any other particles suspended in water, regardless of their nutritional value. Perioral cilia create a continuous flow of water with particles suspended in it in the direction of the oral opening, which is located deep in the peristome. Small food particles (most often bacteria) penetrate through the mouth into a small tube-shaped pharynx and accumulate at the bottom, at the border with the endoplasm. The mouth opening is always open. Perhaps it would not be a mistake to say that the ciliate slipper is one of the most voracious animals: it feeds continuously. This process is interrupted only at certain moments in life associated with reproduction and the sexual process.
The food lump that has accumulated at the bottom of the pharynx subsequently breaks away from the bottom of the pharynx and, together with a small amount of liquid, enters the endoplasm, forming a digestive vacuole. The latter does not remain at the site of its formation, but, entering the endoplasmic currents, makes a rather complex and natural path in the body of the shoe, called cyclosis of the digestive vacuole (Fig. 88). During this rather long (at room temperature taking about an hour) journey of the digestive vacuole, a number of changes occur inside it related to the digestion of the food contained in it.
Here, as in amoebas and some flagellates, typical intracellular digestion occurs. From the endoplasm surrounding the digestive vacuole, digestive enzymes enter it and act on food particles. The products of food digestion are absorbed through the digestive vacuole into the endoplasm.
As the cyclosis of the digestive vacuole progresses, several phases of digestion change in it. In the first moments after the formation of a vacuole, the liquid filling it differs little from the surrounding liquid. Soon, digestive enzymes begin to flow from the endoplasm into the vacuole and the reaction of the environment inside it becomes sharply acidic. This can be easily detected by adding some indicator to the food, the color of which changes depending on the reaction (acidic, neutral or alkaline) of the environment. The first phases of digestion take place in this acidic environment. Then the picture changes and the reaction inside the digestive vacuoles becomes slightly alkaline. Under these conditions, further stages of intracellular digestion take place. The acidic phase is usually shorter than the alkaline phase; it lasts approximately 1/6-1/4 of the entire period of residence of the digestive vacuole in the body of the ciliate. However, the ratio of acidic and alkaline phases can vary over a fairly wide range depending on the nature of the food.
The path of the digestive vacuole in the endoplasm ends with it approaching the surface of the body and through the pellicle its contents, consisting of liquid and undigested food debris, are thrown out - defecation occurs. This process, unlike amoebas, in which defecation can occur anywhere, in slippers, as well as in other ciliates, is strictly confined to a specific area of the body located on the ventral side (the abdominal surface is conventionally called the surface of the animal on which the perioral recess is located ), approximately midway between the peristome and the posterior end of the body.
Thus, intracellular digestion is a complex process consisting of several phases that successively replace each other.

Calculations show that in approximately 30-45 minutes, the slipper excretes a volume of liquid through contractile vacuoles equal to the volume of the ciliate’s body. Thus, thanks to the activity of contractile vacuoles, a continuous flow of water occurs through the body of the ciliate, entering from the outside through the mouth opening (together with the digestive vacuoles), as well as osmotically directly through the pellicle. Contractile vacuoles play an important role in regulating the flow of water passing through the body of the ciliate and regulating osmotic pressure. This process here proceeds in principle the same way as in amoebas, only the structure of the contractile vacuole is much more complex.
For many years, among scientists involved in the study of protozoa, there has been a debate about whether there are any structures in the cytoplasm associated with the appearance of a contractile vacuole, or whether it is formed anew every time. On a living ciliate, no special structures that would precede its formation can be observed. After the contraction of the vacuole - systole - occurs, absolutely no structures are visible in the cytoplasm in the place of the former vacuole. Then a transparent vesicle or afferent canals reappear and begin to increase in size. However, no connection between the newly emerging vacuole and the previously existing one is detected. It seems that there is no continuity between successive cycles of contractile vacuoles and that each new contractile vacuole is formed anew in the cytoplasm. However, special research methods have shown that this is actually not the case. The use of electron microscopy, which provides a very high magnification (up to 100 thousand times), convincingly showed that ciliates in the area where contractile vacuoles are formed have a particularly differentiated cytoplasm, consisting of an interweaving of very thin tubes. Thus, it turned out that the contractile vacuole does not appear in the cytoplasm in an “empty place”, but on the basis of a previous special cell organelle, the function of which is the formation of a contractile vacuole.
Like all protozoa, ciliates have a cell nucleus. However, in the structure of the nuclear apparatus, ciliates differ sharply from all other groups of protozoa.
The nuclear apparatus of ciliates is characterized by its dualism. This means that ciliates have two different types of nuclei - large nuclei, or macronuclei, and small nuclei, or micronuclei. Let's see what structure the nuclear apparatus has in the ciliate slipper (Fig. 85).
In the center of the body of the ciliate (at the level of the peristome) there is a large massive ovoid or bean-shaped nucleus. This is a macronucleus. In close proximity to it there is a second nucleus, many times smaller in size, usually quite closely adjacent to the macronucleus. This is a micronucleus. The difference between these two nuclei is not limited to size; it is more significant and deeply affects their structure.
The macronucleus, compared to the micronucleus, is much richer in the special nuclear substance (chromatin, or, more precisely, deoxyribonucleic acid, abbreviated DNA) that is part of the chromosomes.
Research in recent years has shown that the macronucleus has several tens (and in some ciliates hundreds) times more chromosomes than micronuclei. Macronucleus is a very unique type of multichromosomal (polyploid) nuclei. Thus, the difference between micro- and macronuclei affects their chromosomal composition, which determines the greater or lesser richness of their nuclear substance - chromatin.
In one of the most common types of ciliates - shoes(Paramecium caudatum) - there is one macronucleus (abbreviated Ma) and one micronucleus (abbreviated Mi). This structure of the nuclear apparatus is characteristic of many ciliates. Others may have several Ma and Mi. But a characteristic feature of all ciliates is the differentiation of nuclei into two qualitatively different groups, Ma and Mi, or, in other words, the phenomenon of nuclear dualism.
How do ciliates reproduce? Let us again turn to the ciliate slipper as an example. If you plant a single specimen of a shoe in a small vessel (microaquarium), then within a day there will be two, and often four, ciliates there. How does this happen? After a period of active swimming and feeding, the ciliate becomes somewhat elongated in length. Then, exactly in the middle of the body, an ever-deepening transverse constriction appears (Fig. 90). In the end, the ciliate is, as it were, laced in half and from one individual two are obtained, initially somewhat smaller in size than the mother individual. The entire division process takes about an hour at room temperature. The study of internal processes shows that even before the transverse constriction appears, the process of fission of the nuclear apparatus begins. Mi is the first to share, and only after that is Ma. We will not dwell here on a detailed examination of the processes of nuclear division and will only note that Mi is divided by mitosis, while the division of Ma in appearance resembles direct division of the nucleus - amitosis. This asexual process of reproduction of the slipper ciliates, as we see, is similar to the asexual reproduction of amoebas and flagellates. In contrast, ciliates in the process of asexual reproduction always divide transversely, whereas in flagellates the division plane is parallel to the longitudinal axis of the body.
During division, a deep internal restructuring of the body of the ciliate occurs. Two new peristomes, two pharynxes and two oral openings are formed. At the same time, the division of the basal nuclei of the cilia occurs, due to which new cilia are formed. If the number of cilia did not increase during reproduction, then as a result of each division the daughter individuals would receive approximately half the number of cilia of the mother, which would lead to complete “baldness” of the ciliates. This doesn't actually happen.

From time to time, in most ciliates, including slippers, a special and extremely peculiar form of the sexual process is observed, which is called conjugation. We will not analyze in detail here all the complex nuclear changes that accompany this process, but will note only the most important things. Conjugation proceeds as follows (Fig. 91). Two ciliates come closer, closely attach to each other with their ventral sides and in this form swim together for quite a long time (in a slipper for about 12 hours at room temperature). The conjugants then separate. What happens in the body of the ciliate during conjugation? The essence of these processes comes down to the following (Fig. 91). The large nucleus (macronucleus) is destroyed and gradually dissolves in the cytoplasm. Micronuclei first divide, and some of the nuclei formed as a result of division are destroyed (see Fig. 91). Each conjugant retains two nuclei. One of these nuclei remains in place in the individual in which it was formed (stationary nucleus), while the other actively moves to the conjugation partner (migrating nucleus) and merges with its stationary nucleus. Thus, in each of the conjugants at this stage there is one nucleus, formed as a result of the fusion of stationary and migrating nuclei. This complex nucleus is called a synkaryon. The formation of a synkaryon is nothing more than the process of fertilization. And in multicellular organisms, an essential moment of fertilization is the fusion of the nuclei of germ cells. In ciliates, sex cells are not formed; there are only sex nuclei, which merge with each other. Thus, mutual cross-fertilization occurs.
Soon after the formation of the synkaryon, the conjugants disperse. In the structure of their nuclear apparatus, at this stage they still differ very significantly from the usual so-called neutral (non-conjugating) ciliates, since they have only one nucleus. Subsequently, due to the synkaryon, the normal nuclear apparatus is restored. The synkaryon divides (one or more times). Part of the products of this division, through complex transformations associated with an increase in the number of chromosomes and enrichment of chromatin, turns into macronuclei. Others retain the structure characteristic of micronuclei. In this way, the nuclear apparatus characteristic and typical of ciliates is restored, after which the ciliates begin asexual reproduction by division.
Thus, the process of conjugation includes two essential biological moments: fertilization and restoration of a new macronucleus due to the synkaryon.
What is the biological significance of conjugation, what role does it play in the life of ciliates? We cannot call it reproduction, because there is no increase in the number of individuals. The questions posed above have served as material for numerous experimental studies carried out in many countries. The main result of these studies is as follows. Firstly, conjugation, like any other sexual process, in which two hereditary principles (paternal and maternal) are combined in one organism, leads to an increase in hereditary variability and hereditary diversity. An increase in hereditary variability increases the adaptive capabilities of the organism to environmental conditions. The second biologically important aspect of conjugation is the development of a new macronucleus due to the division products of the synkaryon and at the same time the destruction of the old one. Experimental data show that it is the macronucleus that plays an extremely important role in the life of ciliates. It controls all the main life processes and determines the most important of them - the formation (synthesis) of protein, which makes up the main part of the protoplasm of a living cell. With prolonged asexual reproduction by division, a kind of “aging” process occurs for the macronucleus, and at the same time for the entire cell: the activity of the metabolic process decreases, and the rate of division decreases. After conjugation (during which, as we have seen, the old macronucleus is destroyed), the level of metabolism and the rate of division are restored. Since during conjugation the process of fertilization occurs, which in most other organisms is associated with reproduction and the emergence of a new generation, in ciliates the individual formed after conjugation can also be considered as a new sexual generation, which arises here as if due to the “rejuvenation” of the old one.
Using the example of the slipper ciliate, we became acquainted with a typical representative of a broad class of ciliates. However, this class is characterized by an extraordinary diversity of species both in structure and in lifestyle. Let's take a closer look at some of the most characteristic and interesting forms.
In ciliates, the cilia evenly cover the entire surface of the body. This is a characteristic feature of the structure (Holotricha). Many ciliates are characterized by a different pattern of development of the ciliary cover. The fact is that the cilia of ciliates are capable of joining together to form more complex complexes. For example, it is often observed that cilia located in one or two rows close to each other join (stick together) together, forming a plate, which, like cilia, is capable of beating. Such lamellar contractile formations are called membranellae (if they are short) or membranes (if they are long). In other cases, cilia arranged in a tight bunch are connected together. These formations - cirri - resemble a brush, the individual hairs of which are stuck together. Various kinds of complex ciliated formations are characteristic of many ciliates. Very often, the eyelash cover does not develop evenly, but only in some areas of the body.
CILATE TRUMPETER (STENTOR POLYMORPHIC)
In fresh waters, species of large beautiful ciliates belonging to family of trumpeters(Stentor). This name fully corresponds to the body shape of these animals, which really resembles a pipe (Fig. 92), wide open at one end. When you first meet live trumpeters, you can notice one feature that is not characteristic of the shoe. At the slightest irritation, including mechanical (for example, tapping a pencil on glass where there is a drop of water with trumpeters), their body contracts sharply and very quickly (in a split second), taking on an almost regular spherical shape. Then, quite slowly (time is measured in seconds), the trumpeter straightens out, taking on his characteristic shape. This ability of the trumpeter to contract quickly is due to the presence of special muscle fibers located along the body and in the ectoplasm. Thus, a muscular system can develop in a single-celled organism.

There are species in the trumpeter genus, some of which are characterized by rather bright colors. Very common in fresh waters blue trumpeter(Stentor coeruleus), which is bright blue. This coloration of the trumpeter is due to the fact that the smallest grains of blue pigment are located in its ectoplasm.
Another species of whelk (Stentor polymorphus) is often green in color. The reason for this coloring is completely different. The green color is due to the fact that small unicellular green algae live and reproduce in the endoplasm of the ciliates, which give the trumpeter’s body its characteristic color. Stentor polymorphus is a typical example of mutually beneficial cohabitation - symbiosis. The whelk and the algae have a mutually symbiotic relationship: the whelk protects the algae living in its body and supplies them with carbon dioxide formed as a result of respiration; For their part, the algae provide the whelk with oxygen, which is released during photosynthesis. Apparently, some of the algae is digested by ciliates, becoming food for the whelk.
Trumpeters swim slowly in the water with their wide end first. But they can also temporarily attach to the substrate with the posterior narrow end of the body, on which a small sucker is formed.
In the body of the trumpeter, one can distinguish a trunk section that expands from back to front and a wide perioral (peristomal) field located almost perpendicular to it. This field resembles an asymmetrical flat funnel, at one edge of which there is a depression - a pharynx leading into the endoplasm of the ciliate. The body of the trumpeter is covered with longitudinal rows of short cilia. Along the edge of the peristomal field in a circle there is a powerfully developed perioral (adoral) zone of membranellae (Fig. 92). This zone consists of a large number of individual ciliated plates, each of which in turn is composed of many cilia stuck together, located in two closely adjacent rows of cilia.

In the area of the oral opening, the perioral membranellae are folded toward the pharynx, forming a left-handed spiral. The flow of water caused by the vibration of the perioral membranella is directed towards the oral opening (into the depth of the funnel formed by the anterior end of the body). Along with the water, food particles suspended in the water also enter the pharynx. The food items of the whelk are more varied than those of the slipper. Along with bacteria, it eats small protozoa (for example, flagellates), unicellular algae, etc.
The whelk has a well-developed contractile vacuole, which has a unique structure. The central reservoir is located in the anterior third of the body, slightly below the oral opening. Two long adductor canals extend from it. One of them goes from the reservoir to the posterior end of the body, the second is located in the peristomal field parallel to the perioral zone of the membranellae.
The trumpet ciliate is a favorite subject for experimental studies on regeneration. Numerous experiments have proven the high regenerative ability of trumpeters. A ciliate can be cut into many parts with a thin scalpel, and each of them in a short time (several hours, sometimes a day or more) will turn into a proportionally built, but small trumpeter, which then, as a result of vigorous feeding, reaches the size typical for this species. To complete the restoration processes, the regenerating piece must have at least one segment of a clear-shaped macronucleus.
The trumpeter, as we have seen, has different cilia: on the one hand, short ones that cover the entire body, and on the other, the perioral membrane zone. In accordance with this characteristic structural feature, the order of ciliates to which the trumpeter belongs received the name heterociliated ciliates(Heterotricha).
Ciliate BURSARIA (BURSARIA TRUNCATELLA)
The second interesting representative of heterociliated ciliates - often found in fresh waters bursaria(Bursaria truncatella, Fig. 93). This is a giant among ciliates: its dimensions can reach 2 mm, the most common values are 0.5-1.0 mm. Bursaria is clearly visible to the naked eye. In accordance with its name, the bursaria has the shape of a bag, wide open at the anterior end (bursa is a Latin word translated into Russian as “purse”, “bag”) and somewhat expanded at the posterior end. The entire body of the ciliate is covered with longitudinally running rows of short cilia. Their beating causes the rather slow forward movement of the animal. Bursaria swims as if “waddling” from side to side.

From the anterior end deep into the body (approximately 2/3 of its length) a perioral depression, the peristome, protrudes. On the ventral side it communicates with the external environment through a narrow gap; on the dorsal side, the peristome cavity does not communicate with the external environment. If you look at the cross section of the upper third of the body of the bursaria (Fig. 93, B), you can see that the peristome cavity occupies most of the body, while the cytoplasm surrounds it in the form of a rim.
At the anterior end of the body, on the left, the very powerfully developed zone of perioral (adoral) membranella in Bursaria originates (Fig. 93, 4). It descends into the depths of the peristome cavity, turning to the left. The adoral zone extends to the deepest part of the peristome. Apart from the perioral membranellae, there are no other ciliated formations in the peristome cavity, with the exception of the ciliated strip running along the ventral side of the peristome cavity (Fig. 93, 10). On the inner posterior wall of the peristomal cavity there is a narrow gap running almost along its entire length (Fig. 93, 7), the edges of which are usually closely adjacent to each other. This is the mouth slit. Its edges move apart only at the time of eating.

Bursaria do not have a narrow food specialization, but are mainly predators. As they move forward, they encounter various small animals. Thanks to the work of membranellae in the perioral zone, prey is forcefully drawn into the vast peristomal cavity, from where it can no longer swim out. Food objects are pressed against the dorsal wall of the peristomal cavity and penetrate the endoplasm through the expanding oral slit. Bursaria are very voracious, they can swallow quite large objects: for example, their favorite food is slipper ciliates. Bursaria is capable of swallowing 6-7 shoes in a row. As a result, very large digestive vacuoles are formed in the endoplasm of the bursaria.
The nuclear apparatus of the bursaria is quite complex. They have one long, sausage-shaped macronucleus and a large (up to about 30) number of small micronuclei randomly scattered in the endoplasm of the ciliate.
Bursaria are among the few species of freshwater ciliates that lack a contractile vacuole. How osmoregulation is carried out in this large ciliate is still not entirely clear. Under the ectoplasm of the bursaria, in different parts of the body, one can observe liquid bubbles of various shapes and sizes - vacuoles, which change their volume. Apparently, these irregularly shaped vacuoles correspond in their function to the contractile vacuoles of other ciliates.

It is interesting to observe the successive stages of asexual reproduction of Bursaria. At the initial stages of division, a complete reduction of the entire peristome cavity and the perioral zone of membranella occurs (Fig. 94). Only the outer ciliary cover is preserved. The ciliate takes the form of an egg. After this, the body is laced with a transverse groove into two halves. In each of the resulting daughter ciliates, through rather complex transformations, a typical peristome and perioral membrane zone develop. The entire process of dividing the bursaria proceeds quickly and takes a little more than an hour.
It is very easy to observe another important life process in Bursaria, the onset of which is associated with unfavorable conditions for the ciliate - the process of cyst formation (encystation). This phenomenon is characteristic, for example, of amoebas. But it turns out that even such complexly organized protozoa as ciliates are capable of passing into an inactive state. If the culture where the bursaria live is not fed or cooled strongly in time, then within a few hours mass encystment will begin. This process proceeds as follows. Bursarids, just as before division, lose the peristome and perioral zone of membranellae. Then they become completely spherical, after which they secrete a double shell of a characteristic shape (Fig. 94, D).

Bursaria can remain in a state of cysts for months. When favorable conditions occur, the cyst shell bursts, the bursaria come out of it, develop a peristome and begin active life.
STYLONICHIA MYTILUS
Ciliates belonging to the ciliates have a very complex and varied differentiated ciliary apparatus. order of gastrociliformes(Hypotricha), numerous species of which live in both fresh and sea water. One of the most common, frequently encountered representatives of this interesting group can be called stylonychia(Stylonichia mytilus). This is a fairly large ciliate (length up to 0.3 mm), living at the bottom of freshwater reservoirs, on aquatic vegetation (Fig. 95). Unlike the slipper, trumpeter and bursaria, stylonychia does not have a continuous ciliary cover, and the entire ciliary apparatus is represented by a limited number of strictly defined ciliary formations.

The body of stylonychia (like most other gastrociliary ciliates) is strongly flattened in the dorso-ventral direction, and its dorsal and ventral sides, anterior and posterior ends are clearly distinguishable. The body is somewhat widened in front, narrowed in the back. When examining the animal from the ventral side, it is clearly visible that in the anterior third on the left there is a complex peristome and oral opening.
On the dorsal side there are quite sparse cilia that are not capable of beating. They can rather be called thin elastic bristles. They are motionless and have no relation to the function of movement. These cilia are usually credited with a tactile, sensitive function.

All ciliated formations associated with movement and food capture are concentrated on the ventral side of the animal (Fig. 95). There are a small number of thick finger-like structures located in several groups. These are abdominal cirri. Each of them is a complex ciliary formation, the result of the close connection (sticking together) of many dozens of individual cilia. Thus, cirri are like brushes, the individual hairs of which are closely close together and interconnected.
With the help of cirrus, the animal moves quite quickly and “runs” along the substrate. In addition to “crawling” and “running” along the substrate, stylonychia is capable of making rather sharp and strong jumps, immediately breaking away from the substrate. These sharp movements are carried out with the help of two powerful caudal cirri (Fig. 95), which do not take part in normal “crawling”.
Along the edge of the body on the right and left there are two rows of marginal (marginal) cirri. From the right edge of the animal they run along the entire body, while from the left edge they reach only the peristome region. These ciliated formations serve for the forward movement of the animal when it is torn off from the substrate and floats freely in the water.
We see, therefore, that the diverse and specialized ciliary apparatus of stylonychia allows it to make very diverse movements, in contrast to, for example, simple gliding in water, like a slipper or a trumpeter.
The ciliary apparatus associated with the nutritional function is also complex. We have already seen that the perioral recess (peristome), at the bottom of which is the oral opening leading to the pharynx, is located in the front half of the animal on the left. Along the left edge, starting from the very anterior end of the body, there is a highly developed zone of perioral (adoral) membranellas. With their beating, they direct the flow of water towards the mouth opening. In addition, in the area of the peristomal recess there are three more contractile membranes (membranes), with their inner ends extending into the pharynx, and a number of special perioral cilia (Fig. 95). This entire complex apparatus serves to capture and direct food into the mouth.

Stilonychia is one of the protozoa with a very wide range of food items. It can rightfully be called an omnivore. It can feed, like the shoe, on bacteria. Its food items include flagellates and unicellular algae (often diatoms). Finally, stilonychia can also be a predator, attacking other, smaller species of ciliates and consuming them.
Stylonychia has a contractile vacuole. It consists of a central reservoir located at the left posterior end of the peristome, and one posteriorly directed adductor canal.
The nuclear apparatus, as always in ciliates, consists of a macronucleus and a micronucleus.
The macronucleus is composed of two halves connected by a thin constriction; There are two micronuclei, they are located directly near both halves of Ma.
Stilonychia, partly Bursaria, trumpeter - these are all ciliates with a wide range of food items. The ability to absorb various foods is characteristic of most ciliates. However, among them you can also find species that are strictly specialized in terms of the nature of their food.
CILATES-PREDATORS
Among ciliates there are predators that are very “choosy” about their prey. A good example would be ciliates. didinia(Didinium nasutum). Didinium is a relatively small ciliate, with an average length of about 0.1-0.15 mm. The front end is elongated in the form of a proboscis, at the end of which the mouth opening is located. The ciliary apparatus is represented by two corollas of cilia (Fig. 96). Didinium swims quickly in water, often changing direction of movement. The preferred food of didinians is slipper ciliates. In this case, the predator turns out to be smaller than its prey. Didinium penetrates the prey with its trunk, and then, gradually expanding its mouth opening more and more, swallows the shoe whole! The proboscis has a special, so-called rod, apparatus. It consists of a number of elastic, strong rods located in the cytoplasm along the periphery of the proboscis. It is believed that this device increases the strength of the walls of the proboscis, which does not rupture when swallowing prey that is so huge compared to didinium, like a shoe. Didinium is an example of an extreme case of protozoan predation. If we compare didinium's swallowing of its prey - a shoe - with predation in higher animals, then similar examples are difficult to find.

Didinium, having swallowed paramecia, naturally swells greatly. The digestion process is very fast; at room temperature it takes only about two hours. Then the undigested remains are thrown out and didinium begins to hunt for its next victim. Special studies have found that the daily “diet” of didinium is 12 shoes - a truly colossal appetite! It must be borne in mind that in the intervals between the next “hunts” didinia sometimes divide. With a lack of food, didinia very easily encyst and just as easily emerge from the cysts again.
HERBIVOROUS CILATES
Much less common than predation among ciliates is “pure vegetarianism” - feeding exclusively on plant foods. One of the few examples of “vegetarian” ciliates are representatives kind of passula(Nassula). Their food source is filamentous blue-green algae (Fig. 97).

They penetrate the endoplasm through the mouth located on the side, and then are twisted by the ciliates into a tight spiral, which is gradually digested. Algae pigments partially enter the cytoplasm of the ciliate and color it bright dark green.
SUVOIKA (VORTICELLA NEBULIFERA)
An interesting group of ciliates, quite large in number of species, consists of sessile forms attached to the substrate, forming detachment of orbicularis(Peritricha). Widespread representatives of this group are souvoiki(species of the genus Vorticella).
Suvoiki resemble an elegant flower like a bell or lily of the valley, sitting on a long stem, which is attached at its end to the substrate. The suvoika spends most of its life attached to the substrate.

Let's look at the body structure of ciliates. In different species, their sizes vary over a fairly wide range (up to approximately 150 microns). The oral disc (Fig. 98) is located on the expanded anterior part of the body, which is completely devoid of cilia. The ciliary apparatus is located only along the edge of the oral (peristomal) disc (Fig. 98) in a special groove, outside of which a ridge (peristomal lip) is formed. Along the edge of the roller there are three ciliated membranes, two of which are located vertically, one (outer) - horizontally. They form slightly more than one full turn of the spiral. These membranes are in constant flickering motion, directing the flow of water to the mouth opening. The oral apparatus begins as a rather deep funnel at the edge of the peristomal field (Fig. 98), in the depth of which there is an oral opening leading to the short pharynx. Suwoikas, like slippers, feed on bacteria. Their mouth opening is constantly open, and there is a continuous flow of water towards the mouth.
One contractile vacuole without afferent canals is located near the oral opening. The macronucleus has a ribbon or sausage shape, with a single small micronucleus closely adjacent to it.
Suvoika is capable of sharply shortening the stem, which in a split second is twisted like a corkscrew. At the same time, the body of the ciliate contracts: the peristomal disc and membranes are retracted inward and the entire anterior end closes.

The question naturally arises: since the suvoikas are attached to the substrate, how is their distribution throughout the reservoir carried out? This occurs through the formation of a free-swimming stage, the vagrant. A corolla of cilia appears at the posterior end of the body of the ciliate (Fig. 99). At the same time, the peristomal disc is pulled inward and the ciliate is separated from the stalk. The resulting vagrant is able to swim for several hours. Then the events play out in the reverse order: the ciliate attaches to the substrate with its posterior end, a stalk grows, the posterior corolla of cilia is reduced, the peristomal disk at the anterior end straightens, and the adoral membranes begin to work. The formation of vagrants in sowwoy is often associated with the process of asexual reproduction. The ciliate divides on the stalk, and one of the daughter individuals (and sometimes both) becomes a wanderer and swims away.
Many types of suvoek are capable of encysting under unfavorable conditions.
Among the sessile ciliates belonging to the order of oriformes, only relatively few species like the ciliates discussed above are solitary living forms. Most of the species included here are colonial organisms.
Typically, coloniality occurs as a result of incomplete asexual or vegetative reproduction. The individuals formed as a result of reproduction, to a greater or lesser extent, retain connections with each other and together form an organic individuality of a higher order, uniting large numbers of individual individuals, which receives the name colony (we have already met with examples of colonial organisms in flagellate class.

Colonies of round-ciliated ciliates are formed as a result of the fact that separated individuals do not turn into wanderers, but maintain contact with each other using stalks (Fig. 100). In this case, the main stalk of the colony, as well as its first branches, cannot be attributed to any one of the individuals, but belongs to the entire colony as a whole. Sometimes a colony consists of only a small number of individuals, while in other species of ciliates the number of individual individuals in the colony can reach several hundred. However, the growth of any colony is not unlimited. Upon reaching the size characteristic of this species, the colony stops growing and the individuals formed as a result of division develop a corolla of cilia, become wanderers and swim away, giving rise to new colonies.
Colonies of round-ciliated ciliates are of two types. In some stalks, the colonies are irreducible: upon irritation, only individual individuals of the colony contract, drawing in the peristome, but the entire colony as a whole does not undergo changes (this type of colony includes, for example, the genera Epistylis, Opercularia). In others (for example, the genus Carchesium), the stalk of the entire colony is capable of contracting, since the cytoplasm passes inside all the branches and thus connects all individuals of the colony with each other. When such colonies are irritated, they shrink entirely. The entire colony in this case reacts as a single whole, as an organic individuality.
Among all the colonial round-ciliated ciliates, perhaps of particular interest is zootamnia(Zoothamnium arbuscula). The colonies of this ciliate are distinguished by their particularly regular structure. In addition, within the colony there is an interesting biological phenomenon of polymorphism.

A zootamnia colony has the shape of an umbrella. On one main stem of the colony there are secondary branches (Fig. 101). The size of an adult colony is 2-3 mm, so they are clearly visible to the naked eye. Zootamnia live in small ponds with clean water. Their colonies are usually found on underwater plants, most often on elodea (water plague).
The stalks of a zootamnia colony are contractile, since the contractile cytoplasm passes through all branches of the colony, with the exception of the basal part of the main stalk. During the contraction, which occurs very quickly and sharply, the entire colony gathers into a lump.

Zootamnia is characterized by a strictly regular arrangement of branches. One main stalk is attached to the substrate. Nine main branches of the colony extend from its upper part in a plane perpendicular to the stalk, strictly regularly located relative to each other (Fig. 102, 6). Secondary branches extend from these branches, on which individual individuals of the colony sit. Each secondary branch can contain up to 50 ciliates. The total number of individuals in the colony reaches 2-3 thousand.
Most of the individuals of the colony in their structure resemble small solitary suvoeks, 40-60 microns in size. But in addition to small individuals, which are called microzoids, on adult colonies, approximately in the middle of the main branches, individuals of a completely different type and size develop (Fig. 102, 5). These are large spherical individuals with a diameter of 200-250 microns, exceeding in mass the volume of a microzoid by a hundred or more times. Large individuals are called macrozoids.
In their structure, they differ significantly from small individuals of the colony. Their peristome is not expressed: it is retracted inward and does not function. From the very beginning of its development from the microzoid, the macrozoid ceases to take food on its own. It lacks digestive vacuoles. The growth of the macrozoid is obviously carried out due to substances entering through cytoplasmic bridges connecting all individuals of the colony. In the area of the macrozoid’s body with which it is attached to the stalk, there is a cluster of special grains (granules), which, as we will see, play a significant role in its future fate. What are these large spherical macrozoids, what is their biological role in the life of the zootamnia colony? The observation shows that macrozoids are future vagrants from which new colonies develop. Having reached its maximum size, the macrozoid develops a corolla of cilia, separates from the colony and swims away. Its shape changes somewhat; from spherical it becomes conical. After some time, the tramp is attached to the substrate always with the side on which the grain is located. The formation and growth of the stalk begins immediately, and granules, which are localized at the posterior end of the tramp, are spent on the construction of the stalk. As the stem grows, the graininess disappears. After the stalk reaches the final length characteristic of zootamnia, a series of rapidly successive divisions begins, leading to the formation of a colony. These divisions are performed in a strictly defined sequence (Fig. 102).

We will not dwell on the details of this process. Let us just pay attention to the following interesting phenomenon. During the first divisions of zootamnia tramps, during the development of the colony, the peristome and mouth of the forming individuals do not function. Feeding begins later, when the young colony already consists of 12-16 individuals. Thus, all the first stages of colony development are carried out exclusively at the expense of those reserves that were formed in the body of the macrozoid during the period of its growth and development on the mother colony. There is an undeniable similarity between the development of the zootamnia vagrant and the development of the egg in multicellular animals. This similarity is expressed in the fact that development in both cases is carried out at the expense of previously accumulated reserves, without the perception of food from the external environment.
When studying sessile ciliates, the question arises: how do they carry out the form of sexual process characteristic of ciliates - conjugation? It turns out that due to a sedentary lifestyle, it is undergoing some significant changes. By the beginning of the sexual process, special, very small vagrants are formed on the colony. Actively moving with the help of a corolla of cilia, they crawl for some time throughout the colony, and then enter into conjugation with large normal sessile individuals of the colony. Thus, here the differentiation of conjugants into two groups of individuals occurs: small, mobile (microconjugants) and larger, immobile (macroconjugants). This differentiation of conjugants into two categories, one of which (microconjugants) is mobile, was a necessary adaptation to a sedentary lifestyle. Without this, the normal course of the sexual process (conjugation) could obviously not be ensured.
SUCKING CILATES (SUCTORIA)
A very unique group in terms of their way of eating is represented by sucking ciliates(Suctoria). These organisms, like suvoikas and other round-ciliated ciliates, are sessile. The number of species belonging to this order amounts to several dozen. The body shape of sucking ciliates is very diverse. Some of their characteristic species are shown in Figure 103. Some sit on the substrate on more or less long stalks, others do not have stalks, some have a rather strongly branched body, etc. However, despite the variety of shapes, all sucking ciliates are characterized by the following two characteristics :
1) complete absence (in adult forms) of the ciliary apparatus,
2) the presence of special appendages - tentacles, which serve to suck out prey.

Different types of sucking ciliates have different numbers of tentacles. They are often collected in groups. With a high magnification of the microscope, you can see that at the end the tentacle is equipped with a small club-shaped thickening.
How do tentacles function? This question is not difficult to answer by observing sucking ciliates for some time. If any small protozoan (flagellate, ciliate) touches the tentacle of the suctorium, it will instantly stick to it. All attempts by the victim to break away are usually in vain. If you continue to observe the victim stuck to the tentacles, you can see that it gradually begins to decrease in size. Its contents are gradually “pumped” through the tentacles into the endoplasm of the sucking ciliate until only one pellicle remains of the victim, which is discarded. Thus, the tentacles of sucking ciliates are completely unique organs for capturing and at the same time sucking out food, found nowhere else in the animal world (Fig. 103).

Sucking ciliates are immobile predators that do not chase prey, but instantly catch it if only careless prey touches them.

Why do we classify these peculiar organisms as ciliates? At first glance, they have nothing in common with them. The following facts indicate that suctoria belong to ciliates. Firstly, they have a nuclear apparatus typical of ciliates, consisting of a macronucleus and a micronucleus. Secondly, during reproduction they develop cilia, which are absent in “adult” individuals. Asexual reproduction and, at the same time, dispersal of sucking ciliates is carried out through the formation of vagrants equipped with several annular corollas of cilia. The formation of vagrants in suctoria can occur in different ways. Sometimes they are formed as a result of not completely uniform division (by budding), in which each bud that separates outward receives a section of a macronucleus and one micronucleus (Fig. 104, L). Several daughter buds can form on one maternal individual at once (Fig. 104, 5). In other species (Fig. 104, D, E) a very peculiar method of “internal budding” is observed. At the same time, a cavity is formed inside the body of the mother suctoria, in which the wandering bud is formed. It comes out through special holes, through which it “squeezes” with certain difficulty.
This development of the embryo inside the mother's body, and then the act of childbirth, is an interesting analogy of the protozoan with what happens in higher multicellular organisms.
On the previous pages, several typical free-living representatives of the class of ciliates, differently adapted to different environmental conditions, were examined. It is interesting to approach the issue of adaptation of ciliates to living conditions and, on the other hand, to see what are the characteristic general features of ciliates living in certain, sharply defined environmental conditions.
As an example, let's take two very sharply different habitats: life in plankton and life on the bottom in the sand.
PLANKTON CILATES
A fairly large number of species of ciliates are found in both marine and freshwater plankton.
The features of adaptation to life in the water column are especially pronounced in radiolarians. The main line of adaptation to the planktonic lifestyle comes down to the development of such structural features that facilitate the floating of the organism in the water column.

A typical planktonic, and almost exclusively marine family of ciliates is tintinnids(Tintinnidae, Fig. 105, 5). The total number of tintinnid species known so far is about 300. These are small forms, characterized by the fact that the protoplasmic body of the ciliate is placed in a transparent, light and at the same time durable house consisting of organic matter. A disk protrudes from the house, carrying a corolla of cilia, which are in constant flickering motion. In a state of soaring, ciliates in the water column are supported mainly by the constant active work of the ciliary apparatus. The house obviously serves the function of protecting the lower part of the ciliate’s body. Only 2 species of tintinnids live in fresh water (not counting 7 species characteristic only of Lake Baikal).

Freshwater ciliates exhibit some other adaptations to life in plankton. In many of them, the cytoplasm is very strongly vacuolated (Loxodes, Condylostoma, Trachelius), so that it resembles foam. This leads to a significant reduction in specific gravity. All of the listed ciliates also have a ciliated cover, thanks to the work of which the body of the ciliate, whose specific gravity is only slightly higher than the specific gravity of water, is easily maintained in a state of “floating”. In some species, the body shape helps to increase the specific surface area and facilitates soaring in water. For example, some planktonic ciliates of Lake Baikal resemble an umbrella or parachute in shape (Liliomorpha, Fig. 105, 2). There is one planktonic sucking ciliate in Lake Baikal (Mucophrya pelagica, Fig. 105, 4), which differs sharply from its sessile relatives. This species lacks a stalk. Its protoplasmic body is surrounded by a wide mucous sheath - a device leading to weight reduction. Long thin tentacles stick out, which, along with their direct function, probably also perform another - increasing the specific surface area, facilitating soaring in water.
Finally, it is necessary to mention one more, so to speak, indirect form of adaptation of ciliates to life in plankton. This is the attachment of small ciliates to other organisms leading a planktonic lifestyle. So, among round-ciliated ciliates(Peritricha) there are quite numerous species that attach to planktonic copepods. This is a common and normal way of life for these types of ciliates.
Along with the round-ciliated ciliates and among sucking(Suctoria) there are species that settle on planktonic organisms.
CILATES LIVING IN THE SAND
Sandy beaches and shallows provide an extremely unique habitat. Along the sea coast they occupy vast spaces and are characterized by a unique fauna.
Numerous studies carried out in recent years in various countries have shown that the thickness of many sea sands is very rich in a variety of microscopic or approaching microscopic fauna in size. Between the sand particles there are numerous small and minute spaces filled with water. It turns out that these spaces are richly populated by organisms belonging to the most diverse groups of the animal world. Dozens of species of crustaceans, annelids, roundworms, especially numerous flatworms, some mollusks, and coelenterates live here. Protozoa, mainly ciliates, are also found here in large numbers. According to modern data, the fauna of ciliates inhabiting the thickness of sea sands includes approximately 250-300 species. If we take into account not only ciliates, but also other groups of organisms inhabiting the sand layer, then the total number of species will be very large. The entire set of animals inhabiting the thickness of sand, living in the smallest gaps between grains of sand, is called psammophilic fauna.
The richness and species composition of psammophilous fauna is determined by many factors. Among them, the size of sand particles is particularly important. Coarse sands have a poor fauna. The fauna of very fine-grained silted sands (with a particle diameter of less than 0.1 mm), where, obviously, the gaps between the particles are too small for animals to live in, is also poor. Medium- and fine-grained sands are richest in life.
The second factor that plays an important role in the development of psammophilic fauna is the richness of sand in organic remains, decomposing organic substances (the so-called degree of saprobity). Sands devoid of organic matter are poor in life. On the other hand, sands that are very rich in organic matter are almost lifeless, since the decay of organic matter leads to depletion of oxygen. Often anaerobic hydrogen sulfide fermentation is added to this.
The presence of free hydrogen sulfide is an extremely negative factor affecting the development of fauna.
In the surface layers of sand, a rather rich flora of unicellular algae (diatoms, peridinia) sometimes develops. This is a factor favorable to the development of psammophilic fauna, since many small animals (including ciliates) feed on algae.
Finally, a factor that has a very negative effect on the psammophilous fauna is the surf. This is quite understandable, since the surf, washing away the upper layers of sand, kills all living things here. The richest psammophilic fauna is in protected, well-warmed bays. Ebbs and flows do not prevent the development of psammophilous fauna. When at low tide the water temporarily leaves, exposing the sand, then in the thickness of the sand, in the spaces between the grains of sand, it is preserved, and this does not interfere with the existence of animals.
In the ciliates that are part of the psammophilous fauna and belonging to various systematic groups (orders, families), many common features are developed in the process of evolution, which are adaptations to the unique conditions of existence between sand particles.

Figure 106 shows some types of psammophilous fauna of ciliates belonging to different orders and families. There are many similarities between them. The body of most of them is more or less strongly elongated in length, worm-like. This makes it possible to easily “squeeze” into the smallest holes between grains of sand. In many species (Fig. 106), elongation of the body is combined with its flattening. The ciliary apparatus is always well developed, which allows active movement in narrow spaces with a certain force. Often, cilia develop on one side of the worm-like flattened body, while the opposite side is bare. This feature is probably associated with the pronounced ability of most psammophilic species to adhere (attach) to the substrate very closely and very firmly through the ciliary apparatus (a phenomenon called thigmotaxis). This property allows animals to remain in place in cases where water currents arise in the narrow gaps where they live. In this case, it is probably more advantageous for the side opposite to the one with which the animal attached to the substrate to be smooth.
What do psammophilic ciliates eat? A significant part of the diet of many species consists of algae, especially diatoms. Bacteria serve them as food to a lesser extent. This depends to a large extent on the fact that there are few bacteria in sands that are not heavily polluted. Finally, especially among the largest psammophilous ciliates, there are a considerable number of predatory forms that eat other ciliates belonging to smaller species. Psammophilic ciliates are apparently widespread everywhere.
CILATES APOSTOMATES

Ciliates spirophria(Spirophrya subparasitica) in an encysted state can often be found sitting on a small stalk on small planktonic marine crustaceans (especially often on crustaceans of the genus Idia). While the crustacean is actively swimming in sea water, the spirophria sitting on it do not undergo any changes. For the further development of ciliates, it is necessary that the crustacean be eaten by a marine hydroid polyp, which often happens (Fig. 107). As soon as the spirophria cysts, together with the crustacean, penetrate the digestive cavity, small ciliates immediately emerge from them, which begin to vigorously feed on the food gruel formed as a result of the digestion of the swallowed crustacean. Within an hour, the size of the ciliates increases 3-4 times. However, reproduction does not occur at this stage. Before us is a typical stage of growth of ciliates, which is called the trophont. After some time, along with undigested food remains, the trophont is thrown out by the polyp into sea water. Here, actively swimming, it descends along the body of the polyp to its sole, where it attaches, surrounded by a cyst. This stage of an encysted large ciliate sitting on a polyp is called tomonta. This is the breeding phase. The tomont does not feed, but quickly divides several times in succession (Fig. 107, 7). The result is a whole group of very small ciliates. Their number depends on the size of the tomont, which in turn is determined by the size of the trophont that gave it its origin. Small ciliates formed as a result of the division of the tomont (they are called tomites or vagrants) represent the dispersal stage.
They emerge from the cyst and swim quickly (without feeding, but using the reserves they have in the cytoplasm). If they are “lucky enough” to come across a copepod, they immediately attach themselves to it and encyst. This is the stage at which we began to consider the cycle.
In the life cycle of spirophria that we have considered, attention is drawn to the sharp demarcation of stages that have different biological significance. Trophon is a stage of growth. It only grows, accumulates a large amount of cytoplasm and all kinds of reserve substances due to energetic and fast nutrition. The trophont is not capable of reproduction. The opposite phenomenon is observed in tomont - the inability to feed and vigorous, rapid reproduction. After each division, no growth occurs, and therefore the reproduction of the tomont is reduced to a rapid disintegration into many strays. Finally, vagrants perform their special and unique function: these are individuals - dispersers and distributors of the species. They are unable to eat or reproduce.
LIFE CYCLE OF ICHTHYOPTHYRIUS


By the end of the growth period, ichthyophthirius reaches a very large size compared to vagrants: 0.5-1 mm in diameter. Upon reaching the maximum size, the ciliates move actively from the tissues of the fish into the water and slowly swim for some time with the help of the ciliary apparatus covering their entire body. Soon, large ichthyophthirius settle on some underwater object and secrete a cyst. Immediately after encystment, successive divisions of the ciliate begin: first in half, then each daughter individual is divided again into two, etc. up to 10-11 times. As a result, up to 2000 small, almost round individuals covered with cilia are formed inside the cyst. Inside the cyst, the wanderers are actively moving. They pierce the shell and come out. Actively swimming vagrants infect new fish.
The rate of division of ichthyophthirius in cysts, as well as the rate of its growth in fish tissues, largely depends on temperature. According to research by various authors, the following figures are given: at 26-27°C the process of development of vagrants in the cyst takes 10-12 hours, at 15-16°C it takes 28-30 hours, at 4-5°C it lasts for 6 -7 days.
The fight against ichthyophthirius presents significant difficulties. The main importance here is preventive measures aimed at preventing the free-floating vagrants in the water from penetrating the tissues of the fish. To do this, it is useful to frequently transplant sick fish into new reservoirs or aquariums and create flow conditions, which is especially effective in the fight against ichthyophthirius.
CILATES TRICHODINES

The entire system of adaptations of trichodins to life on the surface of the host is aimed at not being torn away from the host’s body (which almost always equals death), while maintaining mobility. These devices are very perfect. The body of most trichodins has the shape of a fairly flat disk, sometimes a cap. The side facing the host's body is slightly concave; it forms an attachment sucker. Along the outer edge of the sucker there is a corolla of well-developed cilia, with the help of which the movement (crawling) of the ciliate along the surface of the fish’s body mainly occurs. This corolla corresponds to the corolla found in the vagrant sessile ciliates discussed above. Thus, trichodina can be compared to a tramp. On the ventral surface (on the suction cup) Trichodina has a very complex supporting and attachment apparatus that helps keep the ciliate on the host. Without going into details of its structure, we note that its basis is a complex configuration of a ring made up of separate segments bearing outer and inner teeth (Fig. 109, B). This ring forms an elastic and at the same time durable basis of the abdominal surface, which acts as a suction cup. Different types of trichodynes differ from each other in the number of segments forming a ring, and in the configuration of the outer and inner hooks.

On the side of the body of the trichodina opposite to the disc, the peristome and oral apparatus are located. Its structure is more or less typical for round-ciliated ciliates. The adoral membranes, twisted clockwise, lead into a recess at the bottom of which the mouth is located. The trichodine nuclear apparatus is structured typically for ciliates: one ribbon-shaped macronucleus and one micronucleus located next to it. There is one contractile vacuole.
Trichodins are widespread in all types of water bodies. They are especially often found on fry of different species of fish. During mass reproduction, trichodines cause great harm to fish, especially if they cover the gills in masses. This disrupts the fish's normal breathing.
In order to cleanse fish of trichodynes, it is recommended to take medicinal baths from a 2% solution of table salt or 0.01% solution of potassium permanganate (for fry - for 10-20 minutes).
CILATES OF THE INTESTINAL TRACT OF UNGULATES
From the rumen, food is regurgitated through the mesh into the oral cavity, where it is further chewed (rumination). The newly swallowed chewed food mass through a special tube formed by the folds of the esophagus no longer goes into the rumen, but into the book and from there into the abomasum, where it is exposed to the digestive juices of the ruminant. In abomasum, under conditions of an acidic reaction and the presence of digestive enzymes, ciliates die. When they get there with chewing gum, they are digested.
The number of protozoa in the rumen (as well as in the mesh) can reach colossal values. If you take a drop of the contents of the rumen and examine it under a microscope (when heated, since the ciliates stop at room temperature), then the ciliates literally swarm in the field of view. It is difficult, even under cultural conditions, to obtain such a mass of ciliates. The number of ciliates in 1 cm3 of rumen content reaches a million, and often more. In terms of the entire volume of the scar, this gives truly astronomical figures! The richness of the rumen contents in ciliates largely depends on the nature of the ruminant's food. If the food is rich in fiber and poor in carbohydrates and proteins (grass, straw), then there are relatively few ciliates in the rumen. When carbohydrates and proteins (bran) are added to the diet, the number of ciliates increases sharply and reaches huge numbers. It must be borne in mind that there is a constant outflow of ciliates. When they get into the abomasum along with the chewing gum, they die. The high level of the number of ciliates is supported by their vigorous reproduction.
Even ungulates (horse, donkey, zebra) also have a large number of ciliates in the digestive tract, but their localization in the host is different. Even-toed ungulates do not have a complex stomach, so there is no possibility of protozoa developing in the anterior parts of the digestive tract. But in equids the colon and cecum are very well developed, which are usually filled with food masses and play a significant role in digestion. In this section of the intestine, just as in the rumen and reticulum of ruminants, a very rich fauna of protozoa develops, mainly ciliates, most of which also belong to the order of endodiniomorphs. However, in terms of species composition, the fauna of the rumen of ruminants and the fauna of the large intestine of equids do not coincide.
CILATES OF THE INTESTINAL TRACT OF RUMINANTS
Ciliates are of greatest interest Ofrioscolecid family(Ophryoscolecidae), belonging to order entodiniomorph. A characteristic feature of this order is the absence of a continuous ciliary cover. Complex ciliated formations - cirri - are located at the anterior end of the body of ciliates in the area of the mouth. To these basic elements of the ciliary apparatus, additional groups of cirri can be added, located either at the anterior or posterior end of the body. The total number of species of ciliates of the ofrioscolecid family is about 120.

Figure 110 shows some of the most typical representatives of ophrioscolecids from the rumen of ruminants. The most simply structured ciliates are those of the genus Entodinium (Fig. 110, L). At the anterior end of their body there is one perioral zone of cirri. The anterior end of the body, on which the mouth opening is located, can be retracted inward. Ectoplasm and endoplasm are sharply differentiated. The anal tube, which serves to remove undigested food debris, is clearly visible at the posterior end. Slightly more complex structure anoplodynia(Anoplodinium, Fig. 110, B). They have two zones of the ciliary apparatus - perioral cirri and dorsal cirri. Both are located at the front end. At the posterior end of the body of the species shown in the figure there are long, sharp projections - this is quite typical for many species of ophrioscolecids. It has been suggested that these outgrowths help to “push” ciliates between plant particles filling the rumen.
Kinds Eudiplodynia genus(Eudiplodinium, Fig. 110, B) are similar to anoplodynia, but, unlike them, they have a skeletal supporting plate located on the right edge along the pharynx. This skeletal plate consists of a substance close in chemical nature to fiber, i.e., to the substance that makes up the membranes of plant cells.
U genus polyplastron(Polyplastron, Fig. 110, D, E) further complication of the skeleton is observed. The structure of these ciliates is close to eudiplodynia. The differences boil down primarily to the fact that instead of one skeletal plate, these ciliates have five. Two of them, the largest, are located on the right side, and three, smaller ones, on the left side of the ciliate. The second feature of polyplastron is an increase in the number of contractile vacuoles. Entodynia have one contractile vacuole, Anoplodinia and Eudiplodynia have two contractile vacuoles, and Polyplastron has about a dozen of them.
U epidinium(Epidinium, Fig. 110), which have a well-developed carbohydrate skeleton located on the right side of the body, the dorsal zone of the cirri shifts from the anterior end to the dorsal side. Spikes often develop at the posterior end of ciliates of this genus.
The most complex structure is found genus ofrioscolex(Ophryoscolex), after which the entire family of ciliates is named (Fig. 110, E). They have a well-developed dorsal zone of the cirri, covering about 2/3 of the body circumference and skeletal plates. Numerous spines are formed at the rear end, of which one is usually especially long.
Meet some typical representatives ofrioscolecid shows that within this family there has been a significant increase in the complexity of organization (from endodynia to ofrioscolex).
In addition to ciliates Ofrioscolecid family, in the rumen of ruminants, representatives of what is already known to us are found in small quantities order of equiciliate ciliates. They are represented by a small number of species. Their body is evenly covered with longitudinal rows of cilia; there are no skeletal elements. In the total mass of the ciliate population of the rumen, they do not play a noticeable role, and therefore we will not dwell on their consideration here.
What and how do ophrioscolecids ciliates feed? This issue has been studied in detail by many scientists, especially in detail by Professor V.A. Dogel.

The food of ophrioscolecids is quite diverse, and a certain specialization is observed in different species. The smallest species of the genus Entodinium feed on bacteria, starch grains, fungi and other small particles. Many medium and large ofrioscolecids absorb particles of plant tissue, which make up the bulk of the contents of the rumen. The endoplasm of some species is literally clogged with plant particles. You can see how ciliates attack scraps of plant tissue, literally tear them into pieces and then swallow them, often twisting them into a spiral in their body (Fig. 111, 4). Sometimes you have to observe such pictures (Fig. 111, 2), when the body of the ciliate itself turns out to be deformed due to swallowed large particles.
Ophrioscolecids sometimes exhibit predation. Larger species eat smaller ones. Predation (Fig. 112) is combined with the ability of the same species to feed on plant particles.

How do ciliates penetrate the rumen of a ruminant? What are the routes of infection by ofrioscolecids? It turns out that newborn ruminants do not yet have ciliates in the rumen. They are also absent while the animal is feeding on milk. But as soon as the ruminant switches to plant foods, ciliates immediately appear in the rumen and mesh, the number of which quickly increases. Where do they come from? For a long time it was assumed that rumen ciliates form some kind of resting stages (most likely cysts), which are widely dispersed in nature and, when swallowed, give rise to active stages of ciliates. Further studies showed that ruminant ciliates do not have any resting stages. It was possible to prove that infection occurs by active, mobile ciliates that penetrate the oral cavity when chewing gum is regurgitated. If you examine chewing gum taken from the oral cavity under a microscope, it always contains a large number of actively swimming ciliates. These active forms can easily penetrate into the mouth and further into the rumen of other ruminants from a common drinking vessel, along with grass, hay (which may contain saliva with ciliates), etc. This route of infection has been proven experimentally.
If there are no resting stages in ophrioscolicids, then, obviously, it is easy to obtain “non-infusor” animals by isolating them while they are still feeding on milk. If you do not allow direct contact between growing young animals and ruminants with ciliates, then young animals may be left without ciliates in the rumen. Such experiments were carried out by several scientists in different countries. The result was clear. In the absence of contact between young animals (weaned from their mother during the period of milk feeding) and ruminants with ciliates in the rumen, the animals grow up sterile in relation to ciliates. However, even short-term contact with animals that have ciliates (a common feeding trough, a common drinking bucket, a common pasture) is enough for the fauna of ciliates to appear in the rumen of sterile animals.
Above were the results of experiments on keeping ruminants completely devoid of ciliates in the rumen and mesh. This is achieved, as we have seen, by early isolation of the young. Experiments were carried out on sheep and goats.
In this way, it was possible to carry out observations of “infusorless” animals over a significant period of time (over a year). How does the absence of ciliates in the rumen affect the life of the owner? Does the absence of ciliates have a negative or positive effect on the host? To answer this question, the following experiments were carried out on goats. Twin kids (same litter and same sex) were taken to have more similar material. Then one of the twins of this pair was raised without ciliates in the rumen (early isolation), while the other, from the very beginning of feeding on plant foods, was abundantly infected with many types of ciliates. Both received exactly the same diet and were raised in the same conditions. The only difference between them was the presence or absence of ciliates. In several pairs of kids studied in this way, no differences were found in the course of development of both members of each pair (“infusor” and “non-infusor”). Thus, it can be argued that ciliates living in the rumen and mesh do not have any drastic effect on the vital functions of the host animal.
The above experimental results do not allow, however, to assert that rumen ciliates are completely indifferent to the owner. These experiments were carried out with the host's normal diet. It is possible that under other conditions, under a different nutritional regime (for example, with insufficient feeding), it will be possible to identify the influence of the fauna of protozoa inhabiting the rumen on the host.
Various suggestions have been made in the literature about the possible positive effect of rumen protozoan fauna on the digestive processes of the host. It was indicated that many millions of ciliates, actively swimming in the rumen and crushing plant tissues, contribute to the fermentation and digestion of food masses located in the anterior sections of the digestive tract. A significant number of ciliates that enter the abomasum along with the chewing gum are digested, and the protein, which makes up a significant part of the body of the ciliates, is absorbed. Ciliates, therefore, can be an additional source of protein for the host. It has also been suggested that ciliates contribute to the digestion of fiber, which makes up the bulk of ruminant food, transforming it into a more digestible state.
All these assumptions are unproven, and some of them have been objected to. It was indicated, for example, that ciliates build the protoplasm of their body from proteins that enter the rumen with the host's food. By absorbing plant protein, they convert it into animal protein in their body, which is then digested in rennet. Whether this provides any benefits to the owner remains unclear. All these questions are of great practical interest, since we are talking about the digestion of ruminants - the main objects of animal husbandry. Further research on the role of rumen ciliates in ruminant digestion is highly desirable.
Ophrioscolecids of ruminants, as a rule, have a wide specificity. In terms of species, the population of the rumen and the network of cattle, sheep, and goats is very close to each other. If we compare the species composition of the rumen of African antelopes with that of cattle, then here too about 40% of the total number of species will be common. However, there are quite a few species of ophrioscolecids that are found only in antelope or only in deer. Thus, against the background of the general broad specificity of ophrioscolecids, we can talk about their individual, more narrowly specific species.
CILATES OF THE INTESTINAL HOUSES
Let us now turn to a brief introduction to the ciliates that inhabit the large and cecum of equids.
In terms of species, this fauna, like the rumen fauna of ruminants, is also very diverse. Currently, about 100 species of ciliates living in the large intestine of equines have been described. The ciliates found here, in the sense of their belonging to different systematic groups, are more diverse than the ciliates of the ruminant rumen.

The intestines of horses are home to quite a few species of ciliates belonging to the order Equiciliata, i.e., ciliates in which the ciliary apparatus does not form membranellae or cirri near the oral zone (Fig. 113, 1).
Order entodiniomorph(Entodiniomorpha) is also richly represented in the horse's intestines. While in the rumen of ruminants only one family of endodiniomorphs is found (the ophrioscolecid family), representatives of three families live in the intestines of the horse, the characteristics of which we, however, will not dwell on here, limiting ourselves to only a few drawings of typical horse species (Fig. 113) .

Detailed research by A. Strelkov showed that different types of ciliates are far from evenly distributed along the horse’s large intestine. There are two different groups of species, like two faunas. One of them inhabits the cecum and the abdominal section of the large colon (the initial sections of the large intestine), and the other inhabits the dorsal section of the large colon and small colon. These two species complexes are quite sharply demarcated. There are few species common to these two sections - less than a dozen.
 ,
, 
It is interesting to note that among the numerous species of ciliates that inhabit the large intestine of equids, there are representatives of one genus, which belongs to the sucking ciliates. As we saw above, sucking ciliates(Suctoria) are typical free-living sessile organisms with a very special way of feeding using tentacles (Fig. 103). One of childbirth suctorium adapted to such a seemingly unusual habitat as the large intestine of a horse, for example several species allantosis(Allanthosoma). These very peculiar animals (Fig. 114) do not have a stalk, there are no cilia, the club-shaped tentacles thickened at the ends are well developed.
With the help of tentacles, allanthosomes attach themselves to various types of ciliates and suck them out. Often the prey is many times superior to the predator.
The question of the nature of the relationship between the ciliates of the large intestine of equids and their hosts is still unclear. The number of ciliates can be as large, and sometimes even greater, than in the rumen of ruminants. There is data showing that the number of ciliates in the large intestines of a horse can reach 3 million per 1 cm3. The symbiotic significance suggested by some scientists is even less likely than for rumen ciliates.
The most likely opinion is that they cause some harm to the owner by absorbing a significant amount of food. Some ciliates are carried out with fecal matter, and thus the organic substances (including protein) that make up their body remain unused by the owner.
The question of how equids become infected with ciliates inhabiting the large intestine has not yet been resolved.

The balantidium captures a variety of food particles from the contents of the colon. It feeds especially readily on starch grains. If balantidium lives in the lumen of the human colon, then it feeds on the contents of the intestine and does not have any harmful effects. This is a typical “carrier”, which we became acquainted with when considering the dysenteric amoeba. However, balantidium is less likely than dysenteric amoeba to remain such a “harmless lodger.”
Currently, specialists have well developed various methods that allow the cultivation of balantidia in an artificial environment - outside the host body.

As can be seen from the figure, troglodptella is one of the complex endodiniomorphs. In addition to the perioral zone of the cirri (at the anterior end of the body), it has three more zones of well-developed cirri, ring-shaped covering the body of the ciliate. Troglodytella have a well-developed skeletal apparatus consisting of carbohydrates, covering almost the entire anterior end of the body. The sizes of these peculiar ciliates are quite significant. In length they reach 200-280 microns.
MOUTHEAL CILATES ASTOMATS

Supporting skeletal formations develop mainly at the anterior end of the body, which has to experience mechanical stress and overcome obstacles, pushing through the intestinal lumen between food particles. In species genus radiophria(Radiophrya) at the anterior end on one side of the body (which is conventionally considered the ventral side) there are very strong elastic ribs (spicules) lying in the surface layer of ectoplasm (Fig. 117, B, D, E). In species genus menilella(Mesnilella) also have supporting rays (spicules), which for most of their extent lie in the deeper layers of the cytoplasm (in the endoplasm, Fig. 117, A). Similarly arranged supporting structures are also developed in species of some other genera of astomata.


Asexual reproduction in some ciliates astomat occurs in a unique way. Instead of transverse division in two, characteristic of most ciliates, many astomata undergo uneven division (budding). In this case, the buds that separate at the posterior end remain associated with the mother for some time (Fig. 117, B). The result is a chain consisting of a large front and smaller rear individuals (buds). Subsequently, the buds gradually separate from the chain and begin to exist independently. This peculiar form of reproduction is widespread, for example, in Radiophria, already known to us. The chains of some astomata that arise as a result of budding resemble the chains of tapeworms in appearance. Here we again encounter the phenomenon of convergence.
The nuclear apparatus of the astomat has a structure characteristic of ciliates: a macronucleus, most often ribbon-shaped (Fig. 117), and one micronucleus. Contractile vacuoles are usually well developed. Most species have several (sometimes over a dozen) contractile vacuoles arranged in one longitudinal row.
A study of the distribution of astomat species among different host species shows that most of the astomata are confined to strictly defined host species. Most astomats are characterized by narrow specificity: only one species of animal can serve as their host.

Despite the large number of studies devoted to the study of astomat ciliates, one very important aspect of their biology remains completely unclear: how are these ciliates transmitted from one host individual to another? It has never been possible to observe the formation of cysts in these ciliates.
Therefore, it is suggested that infection occurs actively - in mobile stages.
CILATES OF THE INTESTINES OF SEA URCHINES
Sea urchins are very numerous in the coastal zone of our northern (Barents) and Far Eastern seas (Sea of Japan, Pacific coast of the Kuril Islands). Most sea urchins feed on plant matter, mainly algae, which they scrape from underwater objects with special sharp “teeth” surrounding the mouth opening. The intestines of these herbivorous hedgehogs contain a rich fauna of ciliates. They often develop here in large quantities, and the contents of the intestines of a sea urchin under a microscope are almost as “teeming” with ciliates as the contents of the rumen of ruminants. It must be said that, in addition to the profound differences in the living conditions of the infusoria of the sea urchin and ruminant rumen, there are also some similarities. They consist in the fact that both here and there ciliates live in an environment very rich in plant debris. Currently, over 50 species of ciliates are known that live in the intestines of sea urchins, which are found only in the coastal zone, where urchins feed on algae. At great depths, where algae no longer grow, there are no ciliates in sea urchins.


In terms of lifestyle and feeding habits, most infusoria of sea urchins are herbivorous. They feed on algae, which fill the host's intestines in large quantities. Some species are quite “picky” in their choice of food. For example, strobilidium(Strobilidium, Fig. 118, A) feeds almost exclusively on large diatoms. There are also predators here that eat representatives of other, smaller species of ciliates.

In ciliates from the intestines of sea urchins, unlike astomata, there is no strict association with certain host species. They live in a variety of sea urchin species that feed on algae.
The routes of infection of sea urchins by ciliates have not been studied. However, here it can be assumed with a high degree of probability that it occurs in active free-floating forms. The fact is that ciliates from the intestines of sea urchins can live for a long time (many hours) in sea water. However, they have already become so adapted to life in the intestines of hedgehogs that outside their body, in sea water, they die sooner or later.
Concluding our acquaintance with ciliates, it should be emphasized once again that they represent a species-rich, extensive and prosperous group (class) of the animal world. Remaining at the level of cellular organization, ciliates have achieved the greatest complexity of structure and function compared to other classes of protozoa.
A particularly significant role in this progressive development (evolution) was probably played by the transformation of the nuclear apparatus and the emergence of nuclear dualism (qualitative inequality of nuclei). The richness of the macronucleus in nucleic substances is associated with active metabolic processes, with vigorous processes of synthesis of proteins in the cytoplasm and nuclei.
Conclusion
We have come to the end of our review of the structure and lifestyle of a vast type of animal life - protozoa. Their characteristic feature, as has been repeatedly emphasized above, is unicellularity. In terms of their structure, protozoa are cells. However, they are incomparable to the cells that make up the body of multicellular organisms, because they themselves are organisms. Thus, protozoa are organisms at the cellular level of organization. Some highly organized protozoa, possessing many nuclei, already seem to go beyond the morphological limits of the cell structure, which gives grounds for some scientists to call such protozoa “supracellular”. This changes little the essence of the matter, since the unicellular organization is still typical of Protozoa.
Within the limits of unicellularity, protozoa have gone a long way of evolutionary development and have given rise to a huge variety of forms adapted to a wide variety of living conditions. At the heart of the family tree of protozoa are two classes: sarcodaceae and flagellates. The question of which of these classes is more primitive is still being debated in science. On the one hand, the lower representatives of sarcodes (amoebas) have the most primitive structure. But flagellates exhibit the greatest plasticity in the type of metabolism and stand, as it were, on the border between the animal and plant worlds. In the life cycle of some sarcodae (for example, foraminifera) there are flagellated stages (gametes), which indicates their relationship with flagellates. It is obvious that neither modern sarcodes nor modern flagellates can be the original group in the evolution of the animal world, because they themselves have gone through a long path of historical development and have developed numerous adaptations to modern conditions of life on Earth. Probably, both of these classes of modern protozoa should be considered as two trunks in evolution, originating from ancient forms that have not survived to this day, which lived at the dawn of the development of life on our planet.
In the further evolution of protozoa, changes of various kinds occurred. Some of them led to a general increase in the level of organization, increased activity, and the intensity of life processes. Such phylogenetic (evolutionary) transformations include, for example, the development of organelles for movement and food capture, which reached high perfection in the class of ciliates. It is indisputable that cilia are organelles corresponding (homologous) to flagella. While in flagellates, with few exceptions, the number of flagella is small, in ciliates the number of cilia reaches many thousands. The development of the ciliary apparatus sharply increased the activity of protozoa, made the forms of their relationships with the environment, and the forms of reactions to external irritations more diverse and complex. The presence of a differentiated ciliary apparatus, undoubtedly, was one of the main reasons for the progressive evolution in the class of ciliates, where a wide variety of forms arose, adapted to different habitats.
The development of the ciliary apparatus of ciliates is an example of this kind of evolutionary changes, which were named by Acad. Severtsov aromorphoses. Aromorphoses are characterized by a general increase in organization and the development of devices of wide significance. By increasing organization we mean changes that cause increased vital activity of the body; they are associated with the functional differentiation of its parts and lead to more diverse forms of communication between the organism and the environment. The development of the ciliary apparatus of ciliates refers precisely to this kind of structural transformation in the process of evolution. This is a typical aromorphosis.
In protozoa, as emphasized by V.A. Dogel, changes in the type of aromorphoses are usually associated with an increase in the number of organelles. Polymerization of organelles occurs. The development of the ciliary apparatus in ciliates is a typical example of this type of change. The second example of aromorphosis in the evolution of ciliates can be their nuclear apparatus. We examined above the structural features of the ciliate nucleus. The nuclear dualism of ciliates (the presence of a micronucleus and a macronucleus) was accompanied by an increase in the number of chromosomes in the macronucleus (the phenomenon of polyploidy). Since chromosomes are associated with the main synthetic processes in the cell, primarily with the synthesis of proteins, this process has led to a general increase in the intensity of basic life functions. And here polymerization took place, affecting the chromosomal complexes of the nucleus.
Ciliates- one of the most numerous and progressive groups of protozoa, descended from flagellates. This is evidenced by the complete morphological similarity of their movement organelles. This stage of evolution was associated with two large aromorphoses: one of them affected the organelles of movement, the second - the nuclear apparatus. Both of these types of changes are interconnected, since both lead to increased vital activity and more complex forms of relationships with the external environment.
Along with aromorphoses, there is another type of evolutionary changes, expressed in the development of adaptations (adaptation) to certain, sharply defined conditions of existence. Severtsov called this type of evolutionary changes idioadaptations. In the evolution of protozoa, this type of change played a very important role. Above, when considering different classes of protozoa, numerous examples of idioadaptive changes were given. Adaptations to a planktonic lifestyle in different groups of protozoa, adaptations to life in sand in ciliates, the formation of protective shells of oocysts in coccidia, and much more - all these are idioadaptations that played a large role in the emergence and development of individual groups, but are not associated with general progressive changes in organization .
Adaptations to various specific habitats among protozoa are very diverse. They ensured the widespread distribution of this type in a wide variety of habitats, which was discussed in detail above when describing individual classes.
Great Medical Encyclopedia
Ciliate Tetrahymena thermophila ... Wikipedia
- (Infusoria) class of the most highly developed protozoa (Protozoa). The main features of I.: the presence of cilia (for movement and nutrition), two types of nuclei (polyploid macronucleus and diploid micronucleus, different in structure and... ... Great Soviet Encyclopedia
Or liquid animals (Infusoria) a class of simple animals equipped with ciliated cords or cilia, sometimes replaced in adulthood by cylindrical sucking processes, with a certain body shape, mostly with... ... Encyclopedic Dictionary F.A. Brockhaus and I.A. Efron
Taxonomy of ciliates classification and systematization of organisms belonging to the phylum Ciliates, in accordance with the general concept of taxonomy of protozoa in particular, and animals in general Contents 1 Taxonomy 1.1 Classical ... Wikipedia
Or liquid animals (Infusoria) a class of simple animals equipped with ciliated cords or cilia, sometimes replaced in adulthood by cylindrical sucking processes, with a certain body shape, mostly with... ... Encyclopedia of Brockhaus and Efron
One of the most typical, widely known representatives of ciliates is the slipper ciliate. It lives, as a rule, in stagnant water, as well as in fresh water bodies, where the current is characterized by exceptional pressure. Its habitat must necessarily contain decaying organic matter. It would be advisable to consider in detail all aspects of the life activity of this representative of the fauna.
Representatives of the eyelash

It should be noted that Ciliates are a type whose name comes from the word “tincture” (translated from Latin). This can be explained by the fact that the first representatives of the protozoa were discovered precisely in herbal tinctures. Over time, the development of this type began to rapidly gain momentum. Thus, today about 6-7 thousand species are known in biology, which include the type of Ciliates. If we rely on data from the 1980s, then it can be argued that the type in question contains two classes in its structure: Ciliated ciliates (has three superorders) and Sucking ciliates. In connection with this information, we can conclude that the diversity of living organisms is very wide, which arouses genuine interest.
Type of Ciliates: representatives

Prominent representatives of this type are the balantidium ciliates and the slipper ciliates. Distinctive features of these animals are the covering of the pellicle with cilia, which are used for movement, the protection of ciliates through organs specially designed for this, trichocysts (located in the ectoplasm of the shell), as well as the presence of two nuclei in the cell (vegetative and generative). In addition, the oral cavity on the body of the ciliate forms an oral funnel, which tends to transform into a cellular mouth leading to the pharynx. It is there that digestive vacuoles are created, which serve directly to digest food. But undigested components are removed from the body through powder. Characteristics of the type of ciliates very multifaceted, but the main points are discussed above. The only thing that should be added is that the two ciliates are located in opposite parts of the body. It is through their functioning that excess water or metabolic products are removed from the body.
Ciliate slipper

In order to qualitatively consider the structure and way of life of such interesting organisms of a unicellular structure, it would be advisable to turn to a corresponding example. This requires slipper ciliates, which are widespread in freshwater bodies of water. They can easily be diluted in ordinary containers (for example, in aquariums) by filling the meadow hay with the simplest fresh water, because in tinctures of this type, as a rule, a great many species of protozoa develop, including slipper ciliates. Thus, using a microscope, you can practically study all the information provided in the article.
Characteristics of the slipper ciliate
As noted above, Ciliates are a phylum that includes many elements, the most interesting of which is the slipper ciliate. It is half a millimeter long and has a spindle-shaped shape. It should be noted that visually this organism resembles a shoe, hence the intriguing name. The slipper ciliate is constantly in a state of movement, and it swims with its blunt end first. It is interesting that its movement speed often reaches 2.5 mm per second, which is very good for a representative of this type. On the surface of the body of the slipper ciliate, cilia can be observed, serving as motor organelles. Like all ciliates, the organism in question has two nuclei in its structure: the large one is responsible for nutritional, respiratory, motor and metabolic processes, and the small one takes part in the sexual aspect.
Slipper ciliate organism

The structure of the body of the ciliate slipper is very complex. The outer covering of this representative is a thin elastic shell. She is able to maintain the correct body shape of the body throughout her life. Faithful helpers in this are the perfectly developed supporting fibers located in the cytoplasm layer, which fits tightly to the membrane. The surface of the body of the slipper ciliate is endowed with a huge number (about 15,000) of cilia, oscillating regardless of external circumstances. At the base of each of them there is a basal body. The cilia move approximately 30 times per second, pushing the body forward. It is important to note that the wave-like movements of these instruments are very consistent, which allows the ciliate to slowly and beautifully rotate around the longitudinal axis of its body during movement.
Ciliates - a phylum definitely of interest
For an absolute understanding of all the features of the slipper ciliate, it is advisable to consider the main processes of its life activity. So, it comes down to consuming bacteria and algae. The body of the organism is endowed with a depression called the cellular mouth and passing into the pharynx, at the bottom of which food enters directly into the vacuole. There it is digested for about an hour, making a transition from an acidic to an alkaline environment in the process. Vacuoles move in the body of the ciliate through the flow of cytoplasm, and undigested residues come out in the back of the body through the powder.
Respiration of the ciliate slipper is carried out by the supply of oxygen to the cytoplasm through the integument of the body. And excretory processes occur through two contractile vacuoles. As for the irritability of organisms, ciliates-slippers tend to assemble into bacterial complexes in response to the action of substances secreted by bacteria. And they float away from such an irritant like table salt.
Reproduction

The slipper ciliate can reproduce in one of two ways. Asexual reproduction has become more widespread, according to which the nuclei are divided into two parts. As a result of this operation, each ciliate contains 2 nuclei (large and small). Sexual reproduction is appropriate when there are some deficiencies in nutrition or a change in the temperature of the animal’s body. It should be noted that after this the ciliate can turn into a cyst. But with the sexual type of reproduction, an increase in the number of individuals is excluded. Thus, two ciliates are connected to each other for a certain period of time, as a result of which the shell dissolves and a connecting bridge is formed between the animals. The important thing is that the large nucleus of each of them disappears without a trace, and the small one goes through the fission process twice. Thus, in each ciliate, 4 daughter nuclei are formed, after which three of them are destroyed, and the fourth divides again. This sexual process is called conjugation. And its duration can reach 12 hours.
Type Ciliates, or Ciliates, are the most complex protozoa. On the surface of the body they have organelles of movement - cilia. There are two nuclei in a ciliate cell: the large nucleus is responsible for nutrition, respiration, movement, and metabolism; The small nucleus is involved in the sexual process.
The structural features and vital functions of ciliates are considered using the example of the slipper ciliate.
Habitat, structure and movement. In the same reservoirs where the amoeba proteus and green euglena live, the slipper ciliate is also found (Fig. 30). This unicellular animal, 0.5 mm long, has a spindle-shaped body, vaguely reminiscent of a shoe. The slipper ciliates are always in motion, swimming with the blunt end forward. The movement speed of this animal reaches 2.5 mm per second.
Rice. 30. Structure of the ciliate-slipper: 1 - cilia; 2 - contractile vacuole; 3 - cytoplasm; 4 - large core; 5 - small core; b - cell membrane; 7 - cell mouth; 8 - cell pharynx; 9 - digestive vacuole; 10 - powder
The body of ciliates is more complex than that of amoeba and euglena. The thin elastic shell covering the outside of the ciliate maintains the constant shape of its body. This is also facilitated by the development of well-developed supporting fibers, which are located in the layer of cytoplasm adjacent to the membrane. There are about 15 thousand oscillating cilia located on the surface of the ciliate's body. At the base of each cilium lies a basal body. The movement of each eyelash consists of a sharp stroke in one direction and a slower, smooth return to its original position. The cilia oscillate approximately 30 times per second and, like oars, push the ciliate forward, the wave-like movement of the cilia is coordinated. When a slipper ciliate swims, it slowly rotates around the longitudinal axis of the body.
Under the elastic shell, special formations are scattered throughout the body - trichocysts (from the Greek trichos - “hair” and cystis - “bubble”). These are short “sticks” located in one layer perpendicular to the surface of the body. In case of danger, the trichocysts are thrown out with force, turning into thin, long elastic threads that hit the predator attacking the shoe. Over time, new trichocysts appear in place of used trichocysts.
Nutrition. On the body of the ciliate there is a depression - a cellular mouth, which passes into the cellular pharynx. Thicker and longer cilia are located near the mouth. They push bacteria into the throat along with the flow of water - the shoe's main food. At the bottom of the pharynx, food enters the digestive vacuole. Digestive vacuoles move in the body of the ciliate by a current of cytoplasm. In the vacuole, food is digested, the digested products enter the cytoplasm and are used for life. The undigested residues remaining in the digestive vacuole are thrown out at the posterior end of the body through a special structure - powder.
The slipper ciliate finds its prey by sensing the presence of chemicals that release clusters of bacteria.
Selection. In the body of the slipper ciliate there are two contractile vacuoles, which are located at the anterior and posterior ends of the body. Each vacuole consists of a central reservoir and 5-7 channels directed to these reservoirs. First, the channels are filled with liquid, then it enters the central reservoir, and then the liquid is expelled out. The entire cycle of contraction of these vacuoles occurs once in 10-20 seconds. Contractile vacuoles remove harmful substances that are formed in the body and excess water.
Breath. Like other free-living unicellular animals, in ciliates respiration occurs through the integument of the body.
Reproduction. Sexual process. Slipper ciliates usually reproduce asexually - by dividing in two (Fig. 31, A). However, unlike flagellates, ciliates are divided across the body. The nuclei are divided into two parts, and each new ciliate contains one large and one small kernel. Each of the two daughter ciliates receives part of the organelles (for example, contractile vacuoles), while others are formed anew. Slipper ciliates divide once or twice a day.

Rice. 31. Asexual reproduction (A) and sexual process (B) in the ciliate slipper
During the sexual process, the number of individuals does not increase. Two ciliates are temporarily connected to each other (Fig. 31, B). At the point of contact, the membrane dissolves, and a connecting bridge of cytoplasm is formed between the animals. The large core of each ciliate disappears. The small nucleus divides twice, and four daughter nuclei are formed in each ciliate. Three of them are destroyed, and the fourth is divided again. As a result, two nuclei remain in each ciliate. One of these nuclei of each of the two individuals passes through a cytoplasmic bridge into another ciliate (that is, an exchange of nuclei occurs) and there merges with the remaining nucleus. Then, in each ciliate, a large and small nuclei are formed from this newly formed nucleus, and the ciliates disperse. This sexual process is called conjugation. It lasts about 12 hours.
The sexual process leads to renewal, exchange between individuals and redistribution of hereditary (genetic) material, which increases the vitality of organisms.

Rice. 32. Variety of ciliates: 1 - bursaria; 2 - stentor; 3 - stylonychia; 4 - suvoika
Bursaria has one large and long sausage-shaped core, about 30 small nuclei. Most ciliates actively swim, but some of them, for example stylonychia, move along the bottom of a reservoir, on aquatic plants, as if walking on special elongated cilia located on the ventral side of the body . Other ciliates, such as suvoyki, are attached to the bottom or to plants with long stems, which can contract thanks to special contractile fibers. Many suvoikas form colonies. These ciliates feed mainly on bacteria. Sucking ciliates also lead a sedentary, motionless lifestyle. They lack eyelashes. They are equipped with sucking tentacles in the form of thin contractile tubes, which serve to catch prey (mainly other protozoa) and suck out the contents from it. Protozoa that touch the tentacles, such as flagellates, instantly stick to them. And then the contents of the victim are absorbed, as if pumped along the tentacle into the sucking ciliate.

Rice. 33. Protozoa from the stomach of ungulates
Some ciliates live in the intestines of large herbivorous ungulates (Fig. 33). In cows, sheep, goats, antelopes, and deer, ciliates inhabit the anterior sections of the stomach in huge quantities. These ciliates feed on bacteria, starch grains, fungi, and particles of plant tissue. Larger ciliates devour smaller ones. In other parts of the stomach of herbivores, ciliates are digested. Thus, these ciliates benefit those animals in whose stomachs they live. Infusoria infection occurs during group feeding or watering.
Laboratory work No. 1
- Subject. The structure and movement of the ciliate slipper. Target. To study the features of the structure and movement of the ciliate-slipper.
- Equipment: microscope, tripod magnifying glass, slide and cover glass, pipette, cotton wool, culture of ciliates in vitro.
Progress
- Determine whether the slipper ciliates in the test tube are visible to the naked eye.
- Apply a drop of water with slipper ciliates from a test tube onto a glass slide. Using a magnifying glass, examine the shape of the body, the external structure, the difference between the front and back parts of the body, and the method of movement. Count the number of ciliates in a drop of water.
- Place two drops of water with ciliates on a glass slide and connect them with a water “bridge”. Place a crystal of salt on the edge of one drop. Explain the phenomena occurring.
- Place two or three fibers of cotton wool in a drop of water with ciliates (to slow down the movement of ciliates). Cover carefully with a coverslip.
- Place the specimen under a microscope. Consider first at low and then at high magnification of the microscope what is happening inside the body of the ciliate.
- Draw the external and internal structure of the slipper ciliate using a high-magnification microscope. Make the necessary designation.
- Based on observations, list the characteristics characteristic of ciliates as representatives of protozoa.
Ciliates are complexly organized protozoa. They have two nuclei in the cell: large and small. They reproduce asexually and sexually. Sexual reproduction promotes renewal, exchange between individuals and redistribution of hereditary (genetic) material, which increases the vitality of ciliates.
Exercises based on the material covered
- Why is the slipper ciliate so named?
- What signs prove the more complex organization of the slipper ciliate compared to amoeba Proteus and Euglena green?
- How does the ciliate-slipper structure, which is more complex than that of other protozoa, manifest itself in the processes of nutrition and excretion?
- What are the features of the reproduction process of the slipper ciliates?
- Why is the sexual process important biologically in the life of the slipper ciliate?
To type ciliates include about 7 thousand species of protozoa, the sizes of which range from 0.01 to 3 mm. Their organelles of movement are multiple cilia, which is why the second name of the type is ciliary.
Most species of ciliates have two nuclei. The first is a large vegetative macronucleus. It has a polyploid set of chromosomes and is responsible for the regulation of metabolism, for example, protein synthesis. The second core is small generative, micronucleus. It has a diploid set of chromosomes and takes part in the process of sexual reproduction.
Ciliate slipper
The slipper ciliate has a high title - it is the most complex in structure of all single-celled organisms. Its “sole-shaped” shape is unchanged due to the dense ectoplasm, the outer cytoplasmic layer that forms the additional shell of the body - pellicle. The slipper ciliate is a dirty creature, it lives in fresh water with a high level of organic pollution: something must rot there. By the way, this ciliate feels great in home aquariums, where it feeds fish.
Structure
1. Inside the cytoplasm, in opposite parts of the ciliate, there are two contractile vacuoles. Each is a reservoir with 5-7 afferent tubules. They have the shape of a sun with rays and are perfectly visible under a microscope. Contracting one by one, the vacuoles remove harmful substances and excess water.
2. Two kernels strikingly different in size.
3. Trichocysts- a means of protecting the ciliate-slipper. They are spindle-shaped and attached to membrane sacs. In the event of any irritation of the ciliates, the membrane sacs contract, the trichocysts greatly elongate, and the body seems to bristle with multiple thin threads-needles.
4. The entire body of the ciliate is covered with a huge amount eyelashes- there can be from 10 to 15 thousand of them! They are organized in rows, and the longest are located on the edge of the groove ending in the mouth opening. Thanks to the wave-like movement of the cilia, the ciliate develops a very decent speed for its size - up to 2 mm per second.
5. Cell mouth- the place where the membrane protrudes into the ciliate. Here are the longest and strongest cilia, which help food get into the mouth and further into the mouth. cell pharynx.
Nutrition
1. Through the lower end of the pharynx, food enters the cytoplasm.
2. The food of ciliates is unicellular algae and bacteria. The process of their digestion occurs in digestive vacuoles, which migrate along the entire cell.
3. Undigested food fragments are released through powder, located in the conditional abdominal part of the ciliate.
Unlike sarcodes, the outer layer of the cytoplasm of ciliates is compacted, forming a pellicle, which gives the animal certain shapes characteristic of each type of ciliate. So, for example, the body shape of the paramecium resembles a shoe, in the stentor it looks like a trumpet, and in the suvoika it looks like a bell with a stem. The ciliates bursaria, genu, and didinium are sac- or barrel-shaped, spirostomum is worm-shaped, and pediment is bean-shaped and flattened. Stilonychia and the similar Euplotes also have a flattened body. This variety of forms is associated with the lifestyle and living conditions of each species of ciliates and is the result of divergence in the process of natural selection.
The presence of a dense shell put a certain limit on the unlimited change in body shape in ciliates, which is characteristic of sarcodes. However, ciliates retained the ability to bend, stretch and contract in response to external stimuli, changing the shape of the body. At the same time, the possibility of forming pseudopods, capturing food and excreting excess and harmful metabolic products anywhere in the body has disappeared. All these difficulties were overcome in the process of evolution through the development of various organelles in ciliates. For example, they developed organelles of movement (cilia), food capture (oral opening and pharyngeal canal), excretion (fixed pulsating vacuoles), protection (trichocysts), and the nuclear apparatus also became more complex (the presence of large and small nuclei) and internal differentiation of the cytoplasm (contractile fibers - myonemes - and some other fine structures appeared).
Of the various transformations that occurred in the body of ciliates, the greatest significance in increasing their vital activity was, firstly, the doubling of the nuclear apparatus with the division of functions between the large and small nuclei, and secondly, the development of the ciliary cover. On the basis of these two main aromorphoses, ciliates underwent a process of adaptation to various living conditions, which led to a variety of forms such as idioadaptations.
Types of ciliates
Currently, about 6,000 species of ciliates are known, widely distributed in various habitats. For example, they live in sea and fresh water, in soil, in mosses, on the bark of trees and on the surface of rocks, on the outer covers of animals (sponges, bryozoans, worms, insects, crustaceans, amphibians, fish, etc.), inside stomach of ruminants and ungulates, in the intestines of frogs, hedgehogs, elephants, monkeys, humans, etc. In recent years, the important role of shelled ciliates of sea bells, which live in the surface layer of water (up to 5 cm thick) and are part of the food of juveniles, has become clear fish, worm larvae, mollusks, barnacles and copepods and other inhabitants of the neuston.
The external environment has a diverse impact on the body of ciliates, which respond to the influence of various factors with appropriate movements (taxis). Ciliates, like amoebas, react to light, temperature, chemical, electrical, tactile and other influences. However, the reaction of ciliates to external environmental factors depends both on the changing state of the organism and on the previous conditions of existence. In other words, taxis do not exclude individual behavior, which may reflect the past experience of a given individual. This is evidenced by many experiments conducted on various ciliates.
It is interesting to note that among ciliates there is one species, individuals of which contain chlorophyll in their body. This is a green souvoika.
Defensive reactions in ciliates can be of a varied nature: swimming away, contracting, releasing substances that repel or are harmful to the enemy. It is known that in some ciliates, under the pellicle, perpendicular to the surface of the body, small rod-shaped bodies - trichocysts - are located. With severe irritation, they are shot out of the body, turning into long elastic threads.
In predatory ciliates, trichocysts serve to kill prey, and in peaceful ciliates, they serve to protect against attack. The loss of trichocysts after their firing is compensated by the formation of new rods, which form in the cytoplasm near the macronucleus and then move to the periphery, located between the cilia.
Encystment should be considered a protective means, since the formation of cysts, like in amoebae, allows one to escape for a long time from the effects of unfavorable living conditions. Ciliates in cysts remain viable for up to 7 years. When water bodies dry out, cysts are transported by birds, aquatic insects and wind to other places where they colonize new water bodies. This explains that ciliates are cosmopolitan.
The normal existence of ciliates is ensured by the functions of the nuclear apparatus, which includes a large nucleus (macronucleus) and a small one (micronucleus). The shape of the macronucleus and the number of micronuclei vary among different species of ciliates. The macronucleus is necessary for the vegetative life of ciliates. Without it, they are deprived of the ability to digest and assimilate food, restore lost parts of the body (regenerate), and carry out normal metabolism. The micronucleus plays an important role in the reproduction process.
In all ciliates, regardless of their structure, division occurs across the body and is combined with the regeneration of lost parts. Long-term asexual reproduction ultimately leads ciliates to decrepitude as a result of aging of the macronucleus. The direct consequence of this is a decrease in the level of all body functions, especially metabolism. Conjugation, as it were, rejuvenates the body of ciliates, leading to the replacement of the old macronucleus with a new one and, consequently, to the restoration of normal life activity.
Of great importance for ciliates is their ciliary apparatus, which was modified in the process of evolution by adapting to various living conditions and contributed to their survival in the struggle for existence. The modern names of the orders into which the class of ciliates is divided reflect the features of their ciliary apparatus. So, for example, according to this characteristic, the orders of equiciliate, heterociliary, gastrociliary, and orbicularis are distinguished.
Ciliate slipper
Tailed slipper (caudatum). Slippers constitute a special genus of paramecium in the order of equiciliate ciliates. This genus unites several species inhabiting fresh water bodies. In terms of their size, they are quite accessible for observation with a magnifying glass and microscope. Many species of slippers live in polluted water, where they feed mainly on bacteria and rotting products of organic matter. To obtain artificial cultures of shoes, you can use hay infusion, milk solution or lettuce infusion (according to recipes from practical guides).
The most common type of the genus Paramecium - the caudate slipper caudatum - reaches a length of 0.1-0.3 cm. It received its name due to the fact that the back of its body is slightly narrowed and ends with a tuft of longer cilia (tail). Although the school textbook provides basic information about the structure and functioning of the shoe, it can be supplemented with some other data for extracurricular work.
Observing the movements of a shoe in a drop of water under a microscope, students should draw their attention to the great maneuverability of ciliates and the ability to bend their body to some degree. At the same time, on shoes located in the free part of the field of view, it is easy to detect the energetic beating of numerous cilia covering their entire body, to notice the asymmetrical structure of these ciliates and their spiral rotation along their longitudinal axis. Along the way, you can tell students that each shoe has up to 10-15 thousand identical cilia (hence the name of the order equiciliata). The eyelashes flap up to 10 times per second. However, these strokes do not occur simultaneously, but sequentially - from front to back, creating waves along the body, which, summed up, form a flow of water and impart forward movement. If you measure the movement in a straight line, the speed will be equal to 2-2.5 mm/s. This means that the shoe in a second swims a distance 10-15 times greater than the length of its body. It must be added that the entire propulsion system of the shoe works extremely economically. As calculations have shown, it spends only 0.1% of the useful energy received during breathing on movement.
The shoe moves with the front end forward. When faced with an obstacle, she steps back, making coordinated flapping of her eyelashes in the opposite direction. Due to the asymmetry of the body and the helical movement, the shoe at this moment appears at a different angle to the obstacle. Resumption of forward movement leads to a collision at another point of the foreign body. Then follow repeated retreats from it, each time from a different angle. As a result, after some time the shoe deviates so far away from the obstacle that it goes around it. All this can be noticed by the students themselves when observed under a microscope and serve as a starting point for a conversation about the shoe’s reactions to changes in the external environment.
It should be noted that, despite the absence of neurofibrils and other similar organelles in the shoe, its cytoplasm has the ability to perceive changes in the environment and conduct excitation to various parts of the body. Moreover, the body responds to external stimuli as a whole, making appropriate movements.
The most sensitive to touch is the anterior end of the shoe, especially the flagella and cilia of the perioral recess. In this area there is a zone of perception of chemical and thermal (heat) irritations. An encounter with food (bacteria) causes a positive reaction in the shoe, and a negative reaction with inedible objects. These reactions are classified as chemotaxis.
The school textbook describes an experiment using a cookbook and an infusion with bacteria in an experiment with paramecia. This experience shows that the slipper has negative chemotaxis to a certain concentration of sodium chloride and positive chemotaxis to the presence of bacteria that constitute its natural food. Additionally, you can tell students (or conduct an experiment) that a shoe placed in a drop with a 4% formaldehyde solution or a 3% acetic acid solution shoots out trichocysts, i.e., a protective reaction appears in response to a sharp chemical irritation.
An interesting result is obtained if you add a mixture of carmine and sulfur to a drop of water with paramecia. Carried away by the current created by the cilia of the peristome, all these particles first enter the perioral recess, but then they are actively sorted by the slipper. Ultimately, the sulfur is removed from the peristome, but the carmine remains in it.
Observations show that the pulsating vacuoles of the slipper function at different rates, depending on the amount of salts dissolved in the water. For example, at a salinity of 7.5%, the slipper empties pulsating vacuoles approximately every 25 s, and when the salinity drops to 5%, the pulsation accelerates (occurs every 9.3 s). This phenomenon is explained by the fact that the cytoplasm of the shoe contains more salts than the fresh water where it lives. Therefore, her osmotic pressure is higher. The necessary pressure equalization is achieved by the constant absorption of water by the cytoplasm of the paramecium. In this case, excess water is periodically removed through pulsating vacuoles. It is clear that with an increase in the pressure difference between the cytoplasm and water, the process of absorption of water from the outside will increase, and the rate of emptying of vacuoles will correspondingly accelerate. The opposite phenomenon occurs in ciliates that live in sea water, i.e., richer in salts than fresh water. The speed of their pulsations sharply decreases, and in some species, out of two vacuoles, only one remains or even both disappear. According to scientists, a shoe at its optimal temperature (+27°C) releases in 46 minutes. an amount of water equal to the volume of her body.
To show how the slippers react to changes in temperature, the slippers were kept in a horizontal tube, at one end of which the water was heated to a temperature of +30°C, and at the other, it was cooled to +10°C. Ciliates were collected where the temperature was optimal (24 -28°С). The lack of photosensitive elements in the shoe makes it indifferent to this stimulus. Therefore, light signals are not suitable for clarifying the question of the ability of paramecia to form temporary connections.
Some experiments on training ciliates are of interest. For example, Smith trained the paramecium to turn while moving in a capillary tube, where the shoe had to come back when it reached the end. At first she succeeded in this with great difficulty and was accompanied by clumsy movements and changes in the shape of her body when bending in a narrow space. But then, after repeated exercises for 20 hours, the shoe learned to turn more dexterously, spending only 1-2 seconds on this procedure instead of the initial 4-5 minutes. Consequently, the shoe turned out to be capable of improving its movements in an unusually changed environment, showing an individual deviation from typical behavior when solving the life task assigned to it.
Bramstedt's experiments showed that the shoe can also adapt to movement in a circle (in a cylindrical vessel) or move along the edge of a triangular vessel. These movements, acquired through training, were retained by the shoe even if it was transferred to larger vessels of a different shape. So, for example, after learning to move in a circle, a shoe in a trihedral vessel continued to make circular movements, colliding with its walls not in the corners (as untrained shoes did), but in their middle part, as if at the points of contact of an inscribed circle with them. The same shoes that had learned movement in a triangular vessel, after moving them into a more spacious tetrahedral one, retained the acquired character of movement, moving approximately in those directions that corresponded to the contours of the triangle (as if it were inscribed in a quadrangle). Consequently, the shoe “remembered” not only the shape, but also the size of the vessels.
In experiments using an electrical stimulus, it turned out that the shoe has a positive galvanotaxis to the cathode. If on the way of movement the paramecia receives an electric shock in a certain place, it moves away from this point, and then begins to turn back, as if remembering where the injury awaits it.
Thus, in these experiments it is easy to see the possibility of the formation in ciliates of trace reactions similar to the memory of more highly organized animals. However, it is necessary to warn students against mechanically transferring the development of conditioned reflexes in the usual sense to single-celled creatures.
Of great importance in the struggle for existence is the departure of competitive species of slippers into different niches of the same habitat. Interesting in this regard is Gause's experiment on two related species of Paramecia. If you place a mixed population of caudatum and aurelia in a small glass jar, providing them with the same type of yeast as food, then after a while you will see that the tailed slipper will concentrate closer to the surface, while the eared shoe will stay closer to the bottom: This demarcation is possible due to the fact that one of the competitors (the ear) is less whimsical than the other (the tailed one), and can withstand a higher concentration of waste products near the bottom, where it goes.