pure substancecontains particles only one kind. Examples are silver (containing only silver atoms), sulfuric acid, and carbon monoxide ( IV) (contain only the molecules of the corresponding substances). All pure substances have constant physical properties, for example, melting point (T pl ) and boiling point ( T bale ).
A substance is not pure if it contains any amount of one or more other substances -impurities.
Contaminants lower the freezing point and raise the boiling point of a clean liquid. For example, if salt is added to water, the freezing point of the solution will decrease.
Mixes consist of two or more substances. Soil, sea water, air are all examples of different mixtures. Many mixtures can be separated into their component parts − Components - based on the difference in their physical properties.
Traditional methods that are used in laboratory practice to separate mixtures into individual components are:
filtration,
settling followed by decantation,
separation using a separating funnel,
centrifugation,
evaporation,
crystallization,
distillation (including fractional distillation),
chromatography,
sublimation and others.
Filtration. Filtration is used to separate liquids from fine solid particles suspended in it.(fig.37) , i.e. filtering liquid through finely porous materials -filters, which allow liquid to pass through and retain solid particles on their surface. The liquid that has passed through the filter and freed from the solid impurities in it is called filtrate.
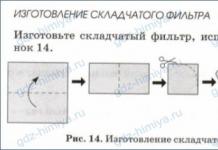
Often used in laboratory practicesmooth and folded paper filters(fig.38) made from non-glued filter paper.
To filter hot solutions (for example, for the purpose of recrystallization of salts), a specialhot filter funnel(fig.39) with electric or water heating).
Often usedvacuum filtration. Vacuum filtration is used to speed up filtration and more completely free the precipitate from solution. For this purpose, a vacuum filtration device is assembled. (fig.40) . It consists ofBunsen flask, Buchner porcelain funnel, safety bottle and vacuum pump(usually water jet).
In the case of filtering a suspension of a sparingly soluble salt, the crystals of the latter can be washed with distilled water on a Buchner funnel to remove the initial solution from their surface. For this purpose, use washer(fig.41) .
Decantation. Liquids can be separated from insoluble solidsdecantation(fig.42) . This method can be used if the solid has a higher density than the liquid. For example, if river sand is added to a glass of water, then when settling, it will settle to the bottom of the glass, because the density of sand is greater than that of water. Then the water can be separated from the sand by simply draining. This method of settling and subsequent draining of the filtrate is called decantation.
Centrifugation.D To accelerate the process of separating very small particles that form stable suspensions or emulsions in a liquid, the method is used. centrifugation. This method can be used to separate mixtures of liquid and solid substances that differ in density. The division is carried out in manual or electric centrifuges(fig.43) .
Separation of two immiscible liquids, having different densities and not forming stable emulsions,can be done with a separating funnel (fig.44) . So you can separate, for example, a mixture of benzene and water. Benzene layer (density = 0.879 g/cm 3 ) located above a layer of water, which has a high density ( = 1.0 g/cm 3 ). By opening the stopcock of the separating funnel, you can carefully drain the bottom layer and separate one liquid from another.
Evaporation(fig.45) - this method involves the removal of a solvent, such as water, from a solution by heating it in an evaporating porcelain dish. In this case, the evaporated liquid is removed, and the dissolved substance remains in the evaporating dish.
Crystallization- this is the process of separating crystals of a solid when a solution is cooled, for example, after it has been evaporated. It should be borne in mind that large crystals are formed when the solution is slowly cooled. Upon rapid cooling (eg cooling under running water), fine crystals form.
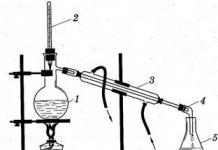
Distillation- a method of cleaning a substance based on the evaporation of a liquid when heated, followed by condensation of the resulting vapors. Purification of water from salts (or other substances, for example, dyes) dissolved in it by distillation is called distillation, and the purified water itself is distilled.
Fractional distillation(fig.46) used to separate mixtures of liquids with different boiling points. A liquid with a lower boiling point boils faster and passes through fractional column(ordephlegmator). When this liquid reaches the top of the fractionation column, it enters thefridge, cooled by water andallongegoing toreceiver(flask or test tube).
Fractional distillation can separate, for example, a mixture of ethanol and water. Boiling point of ethanol 78 0 C, and water 100 0 C. Ethanol evaporates more easily and enters the receiver through the condenser first.
Sublimation - This method is used to purify substances capable of changing from a solid state to a gaseous state when heated, bypassing the liquid state. Further, the vapors of the substance to be purified are condensed, and impurities that are not able to sublimate are separated.
1. Fill in the gaps in the text using the words "components", "differences", "two", "physical".
A mixture can be prepared by mixing at least two substances. Mixtures can be separated into individual components using physical methods based on differences in the physical properties of the components.
2. Complete the sentences.
a) The settling method is based on The fact that the solid particles are large enough, they quickly settle to the bottom, and the liquid can be carefully drained from the sediment.
b) The centrifugation method is based on the action of centrifugal force - heavier particles settle, and the light ones are on top.
c) The filtering method is based on passing a solution of a solid through a filter, where the solid particles are retained on the filter.
3. Insert a missing word:
a) flour and granulated sugar - a sieve; sulfur and iron filings - a magnet.
b) water and sunflower oil - separating funnel; water and river sand - filter.
c) air and dust - respirator; air and poisonous gas - absorbent.
4. Make a list of required filtration equipment.
a) paper filter
b) a glass with a solution
c) glass funnel
d) clean glass
e) glass rod
f) tripod with foot
5. Laboratory experience. Manufacture of ordinary and folded filters from filter paper or paper napkin.

What do you think, through which filter will the solution pass faster - regular or pleated? Why?
Through the pleated - the contact area of the filtration is larger than that of a conventional filter.
6. Suggest methods for separating the mixtures indicated in Table 16.
Ways to separate some mixtures
7. Home experience. Adsorption of Pepsi-Cola dyes by activated charcoal.
Reagents and equipment: carbonated drink, activated carbon; saucepan, funnel, filter paper, electric (gas) stove.
Progress. Pour half a cup (100 ml) of fizzy drink into a saucepan. Add 5 activated charcoal tablets to the same place. Heat the pan for 10 minutes on the stove. Filter the charcoal. Explain the results of the experiment.
The solution became colorless due to the absorption of coloring matter with activated charcoal.
8. Home experience. Adsorption of odorous vapors by corn sticks.
Reagents and equipment: corn sticks, perfume or cologne; 2 identical glass jars with lids.
Progress. Put a drop of perfume into two glass jars. Put 4-5 corn sticks in one of the jars. Close both jars with lids. Shake the jar containing the corn sticks a little. For what?
To increase the rate of adsorption.
Open both banks. Explain the results of the experiment.
There is no smell in the jar where the corn sticks were, as they adsorbed the smell of perfume.
Pure substances and mixtures. Methods for separating mixtures.In order to establish the properties of a substance, it is necessary to have it in its pure form, but substances in nature do not occur in a pure form. Each substance always contains a certain amount of impurities. A substance that contains almost no impurities is called pure. They work with such substances in a scientific laboratory, a school chemistry room. Note that absolutely pure substances do not exist.
Almost all natural substances, foodstuffs (except for salt, sugar, and some others), building materials, household chemicals, many medicines and cosmetics are mixtures.
Natural substances are mixtures, sometimes consisting of a very large number of different substances. For example, natural water always contains salts and gases dissolved in it. Sometimes a very small impurity content can lead to a very strong change in some properties of a substance. For example, the content in zinc of only hundredths of iron or copper accelerates its interaction with hydrochloric acid hundreds of times. When one of the substances is in the mixture in a predominant amount, the entire mixture usually bears its name.
A component is each substance contained in a mixture.
uniform mixtures.
Add a small portion of sugar to a glass of water and stir until all the sugar is dissolved. The liquid will taste sweet. Thus, the sugar did not disappear, but remained in the mixture. But we will not see its crystals, even when examining a drop of liquid through a powerful microscope.

Rice. 3. Homogeneous mixture (water solution of sugar)
The prepared mixture of sugar and water is homogeneous (Fig. 3); the smallest particles of these substances are evenly mixed in it.
Mixtures in which components cannot be detected with the naked eye are called homogeneous.
Water mixed with sand, chalk or clay freezes at 0 0 C and boils at 100 0 C.
Some types of heterogeneous mixtures have special names: foam (for example, foam, soap suds), suspension (a mixture of water with a small amount of flour), emulsion (milk, well-shaken vegetable oil with water), aerosol (smoke, fog).

Rice. 5. Heterogeneous mixtures:
a - a mixture of water and sulfur;
b - a mixture of vegetable oil and water;
c - a mixture of air and water
There are different ways to separate mixtures. The choice of method for separating a mixture is influenced by the properties of the substances that form this mixture.

Let's take a closer look at each method:
settling- a common method of purifying or liquids from water-insoluble mechanical impurities, or liquid substances that are insoluble in each other, having different densities.
Settling is used in the preparation of water for technological and domestic needs, the treatment of sewage, the dehydration and desalting of crude oil, and in many processes of chemical technology. It is an important stage in the natural self-purification of natural and artificial reservoirs.
Filtration- separation of liquid from solid insoluble impurities in it; liquid molecules pass through the pores of the filter, and large particles of impurities are retained.
Imagine that you have a mixture of river sand and water. Determine the type of mixture. ( heterogeneous). Compare the physical properties of river sand and water. (These are substances that are insoluble in each other, having different densities). Suggest a method for separating this mixture ( filtering).
Magnet action- this is a method of separating inhomogeneous mixtures, when one of the substances in the mixture is able to be attracted by a magnet
Evaporation - this is a method of separating homogeneous mixtures, in this case, a solid soluble substance is released from a solution, when heated, water evaporates, and solid crystals remain.
Distillation (Latin meaning "dropping") – This is a method of separating homogeneous mixtures, in which case liquid mixtures are separated into fractions differing in composition. It is carried out by partial evaporation of the liquid, followed by vapor condensation. The distilled fraction (distillate) is enriched with relatively more volatile (low-boiling) substances, and the non-distilled liquid (distillation residue) is enriched with relatively less volatile (high-boiling) substances.
In the laboratory, distillation is carried out on a special installation (Fig. 6). When a mixture of liquids is heated, the substance with the lowest boiling point boils first. Its vapor leaves the vessel, cools, condenses1, and the resulting liquid flows into the receiver. When this substance is no longer in the mixture, the temperature will begin to rise, and over time, another liquid component will boil. Non-volatile liquids remain in the vessel.

Rice. 6. Laboratory installation for distillation: a - conventional; b - simplified
1 - a mixture of liquids with different boiling points;
2 - thermometer;
3 - water cooler;
4 - receiver
Consider how some methods separation of mixtures.
The filtering process underlies the operation of a respirator, a device that protects the lungs of a person working in a heavily dusty environment. The respirator has filters that prevent dust from entering the lungs (Fig. 7). The simplest respirator is a bandage made of several layers of gauze. A filter that extracts dust from the air is also in the vacuum cleaner.
 Rice. 7. Worker in a respirator
Rice. 7. Worker in a respirator
Draw a conclusion by what methods it is possible to separate a mixture of soluble and insoluble substances in water.
Every substance contains impurities. A substance is considered pure if it contains almost no impurities.
Mixtures of substances are either homogeneous or heterogeneous. In a homogeneous mixture, the components cannot be detected by observation, but in an inhomogeneous mixture it is possible.
Some physical properties of a homogeneous mixture differ from those of the components.
In a heterogeneous mixture, the properties of the components are preserved.
Inhomogeneous mixtures of substances are separated by settling, filtering, sometimes by the action of a magnet, and homogeneous mixtures are separated by evaporation and distillation (distillation).
Pure substances and mixtures
We live among chemicals. We inhale air, and this is a mixture of gases (nitrogen, oxygen and others), we exhale carbon dioxide. We wash ourselves with water - this is another substance, the most common on Earth. We drink milk - a mixture of water with the smallest droplets of milk fat, and not only: there is also milk protein casein, mineral salts, vitamins and even sugar, but not the one with which they drink tea, but a special milk - lactose. We eat apples, which consist of a whole range of chemicals - sugar, malic acid, vitamins... apple, but also any other food. We not only live among chemicals, but we ourselves are made of them. Every person - his skin, muscles, blood, teeth, bones, hair are built of chemicals, like a house of bricks. Nitrogen, oxygen, sugar, vitamins are substances of natural, natural origin. Glass, rubber, steel are also substances, more precisely, materials (mixtures of substances). Both glass and rubber are of artificial origin; they did not exist in nature. Completely pure substances are not found in nature or are very rare.
Each substance always contains a certain amount of impurities. A substance that contains almost no impurities is called pure. They work with such substances in a scientific laboratory, a school chemistry room. Note that absolutely pure substances do not exist.
An individual pure substance has a certain set of characteristic properties (constant physical properties). Only pure distilled water has tmelt = 0 °С, tboil = 100 °С, and has no taste. Sea water freezes at a lower temperature, and boils at a higher temperature, its taste is bitter-salty. The water of the Black Sea freezes at a lower temperature and boils at a higher temperature than the water of the Baltic Sea. Why? The fact is that sea water contains other substances, for example, dissolved salts, i.e. it is a mixture of various substances, the composition of which varies over a wide range, but the properties of the mixture are not constant. The concept of "mixture" was defined in the 17th century. English scientist Robert Boyle: "A mixture is an integral system consisting of heterogeneous components."
Almost all natural substances, food products (except salt, sugar, and some others), many medicinal and cosmetic products, household chemicals, and building materials are mixtures.
Comparative characteristics of a mixture and a pure substance
Each substance contained in a mixture is called a component.
Classification of mixtures
There are homogeneous and heterogeneous mixtures.
Homogeneous mixtures (homogeneous)
Add a small portion of sugar to a glass of water and stir until all the sugar is dissolved. The liquid will taste sweet. Thus, the sugar did not disappear, but remained in the mixture. Ho, we will not see its crystals, even when examining a drop of liquid in a powerful microscope. The prepared mixture of sugar and water is homogeneous; the smallest particles of these substances are evenly mixed in it.
Mixtures in which components cannot be detected by observation are called homogeneous.
Most metal alloys are also homogeneous mixtures. For example, an alloy of gold and copper (used to make jewelry) lacks red copper particles and yellow gold particles.
From materials that are homogeneous mixtures of substances, many items for various purposes are made.
All mixtures of gases, including air, belong to homogeneous mixtures. There are many homogeneous mixtures of liquids.
Homogeneous mixtures are also called solutions, even if they are solid or gaseous.
Let us give examples of solutions (air in a flask, table salt + water, small change: aluminum + copper or nickel + copper).
Heterogeneous mixtures (heterogeneous)
You know that chalk does not dissolve in water. If its powder is poured into a glass of water, then chalk particles can always be found in the resulting mixture, which are visible to the naked eye or through a microscope.
Mixtures in which components can be detected by observation are called heterogeneous.
Heterogeneous mixtures include most minerals, soil, building materials, living tissues, turbid water, milk and other foods, some drugs and cosmetics.
In a heterogeneous mixture, the physical properties of the components are preserved. So, iron filings mixed with copper or aluminum do not lose their ability to be attracted to a magnet.
Some types of heterogeneous mixtures have special names: foam (for example, foam, soap suds), suspension (a mixture of water with a small amount of flour), emulsion (milk, well-shaken vegetable oil with water), aerosol (smoke, fog).
Methods for separating mixtures
In nature, substances exist in the form of mixtures. For laboratory research, industrial production, for the needs of pharmacology and medicine, pure substances are needed.
There are many methods for separating mixtures. They are chosen taking into account the type of mixture, state of aggregation and differences in the physical properties of the components.
Methods for separating mixtures

These methods are based on differences in the physical properties of the components of the mixture.
Consider methods for separating heterogeneous and homogeneous mixtures.
Blend example |
Separation method |
Suspension - a mixture of river sand with water |
settling Separation by settling is based on different densities of the substances. Heavier sand settles to the bottom. You can also separate the emulsion: to separate oil or vegetable oil from water. In the laboratory, this can be done using a separating funnel. Oil or vegetable oil forms the top, lighter layer. As a result of settling, dew falls out of the fog, soot is deposited from smoke, cream is settled in milk. |
A mixture of sand and table salt in water |
Filtration The separation of heterogeneous mixtures by filtration is based on the different solubility of substances in water and on different particle sizes. Only particles of substances commensurate with them pass through the pores of the filter, while larger particles are retained on the filter. So you can separate a heterogeneous mixture of table salt and river sand. Various porous substances can be used as filters: cotton wool, coal, fired clay, pressed glass, and others. The filtering method is the basis for the operation of household appliances, such as vacuum cleaners. It is used by surgeons - gauze bandages; drillers and workers of elevators - respiratory masks. With the help of a tea strainer for filtering tea leaves, Ostap Bender - the hero of the work of Ilf and Petrov - managed to take one of the chairs from Ellochka Ogre ("The Twelve Chairs"). |
A mixture of iron powder and sulfur |
Action by magnet or water Iron powder was attracted by a magnet, but sulfur powder was not. The non-wettable sulfur powder floated to the surface of the water, while the heavy wettable iron powder settled to the bottom. |
A solution of salt in water is a homogeneous mixture |
Evaporation or crystallization The water evaporates and salt crystals remain in the porcelain cup. When water is evaporated from lakes Elton and Baskunchak, table salt is obtained. This separation method is based on the difference in the boiling points of the solvent and the solute. If a substance, such as sugar, decomposes when heated, then the water is not completely evaporated - the solution is evaporated, and then sugar crystals are precipitated from a saturated solution. Sometimes it is required to remove impurities from solvents with a lower boiling point, for example, water from salt. In this case, the vapors of the substance must be collected and then condensed upon cooling. This method of separating a homogeneous mixture is called distillation, or distillation. In special devices - distillers, distilled water is obtained, which is used for the needs of pharmacology, laboratories, and car cooling systems. At home, you can design such a distiller. If, however, a mixture of alcohol and water is separated, then the first to be distilled off (collected in a receiving test tube) is alcohol with tboil = 78 °C, and water will remain in the test tube. Distillation is used to obtain gasoline, kerosene, gas oil from oil. |
Chromatography is a special method for separating components based on their different absorption by a certain substance.
If you hang a strip of filter paper over a vessel with red ink, immersing only the end of the strip in them. The solution is absorbed by the paper and rises along it. But the border of the rise of the paint lags behind the border of the rise of the water. This is how the separation of two substances occurs: water and the coloring matter in the ink.
With the help of chromatography, the Russian botanist M. S. Tsvet was the first to isolate chlorophyll from the green parts of plants. In industry and laboratories, instead of filter paper for chromatography, starch, coal, limestone, and aluminum oxide are used. Are substances always required with the same degree of purification?
For different purposes, substances with different degrees of purification are needed. Cooking water is sufficiently settled to remove impurities and chlorine used to disinfect it. Drinking water must first be boiled. And in chemical laboratories for the preparation of solutions and experiments, in medicine, distilled water is needed, as purified as possible from the substances dissolved in it. Highly pure substances, the content of impurities in which does not exceed one millionth of a percent, are used in electronics, semiconductor, nuclear technology and other precision industries.
Do you know what methods exist for separating mixtures? Do not rush to a negative answer. Many of them you apply in your daily activities.
Pure substance: what is it
Atoms, molecules, substances and mixtures are the basic chemical concepts. What do they stand for? In the table of D. I. Mendeleev there are 118 chemical elements. These are different types of elementary particles - atoms. They differ in mass.
Atoms combine with each other to form molecules, or substances. The latter, when combined with each other, form mixtures. Pure substances have constant composition and properties. These are homogeneous structures. But they can be separated into components through chemical reactions.
Scientists say that pure substances in nature practically do not exist. A small amount of impurities is in each of them. This is because most substances differ in activity. Even metals immersed in water dissolve in it at the ion level.
The composition of pure substances is always constant. It is simply impossible to change it. So, if the amount of carbon or oxygen in a carbon dioxide molecule is increased, it will be a completely different substance. And in the mixture, you can increase or decrease the number of components. This will change its composition, but not the fact of existence.
What is a mixture
A combination of several substances is called a mixture. They can be of two types. If the individual components in the mixture are indistinguishable, it is called homogeneous, or homogeneous. There is another name that is most often used in everyday life - a solution. The components of such a mixture cannot be separated by physical methods. For example, from a saline solution, it will not be possible to mechanically extract the crystals that are dissolved in it. In nature, there are not only liquid solutions. So, air is a gaseous homogeneous mixture, and an alloy of metals is a solid.
In heterogeneous or heterogeneous mixtures, individual particles are visible to the naked eye. They differ from each other in composition and properties. This means that they can be separated from each other purely mechanically. Cinderella perfectly coped with this task, which the evil stepmother forced to separate the beans from the peas.
Chemistry: ways to separate mixtures
A huge number of mixtures are found in everyday life and nature. How to choose the right way to separate them? It must necessarily be based on the physical properties of the individual components. If substances have different boiling points, then evaporation followed by crystallization, as well as distillation, will be effective. Such methods are used to separate homogeneous solutions. To separate heterogeneous mixtures, the difference in other properties of their constituents is used: density, wettability, solubility, size, magnetism, etc.
Physical methods for separating mixtures
When the components of the mixture are separated, the composition of the substances themselves does not change. Therefore, it is impossible to call methods for separating mixtures a chemical process. So, by settling, filtering and applying a magnet, it is possible to separate the individual components mechanically. Various devices are used in the laboratory: separating funnel, filter paper, magnetic stripes. These are methods for separating heterogeneous mixtures.

Screening
This method is perhaps the simplest. Every housewife knows him. It is based on the difference in the sizes of the solid components of the mixture. Sifting is used in everyday life to separate flour from impurities, insect larvae and various contaminants. In agricultural production, cereal grains are thus cleaned of foreign debris. Construction workers are sifting a mixture of sand and gravel.

settling
This method of separation of mixtures is used for components with different densities. If the sand gets into the water, the resulting solution must be mixed well and left for a while. The same can be done with a mixture of water and vegetable oil or oil. The sand will sink to the bottom. But the oil, on the contrary, will be collected from above. This method is observed in everyday life and nature. For example, soot settles from smoke, and separate drops of dew from fog. And if you leave homemade milk for the night, then by morning you can collect the cream.
Filtration
Drinkers of brewed tea use this method daily. We are talking about filtration - a method of separating mixtures based on the different solubility of the components. Imagine that iron filings and salt got into the water. Large insoluble particles will remain on the filter. And the dissolved salt will pass through it. The principle of this method underlies the work of vacuum cleaners, the action of respiratory masks and gauze bandages.

Magnet action
Suggest a method for separating mixtures of sulfur and iron powders. Naturally, this is the action of a magnet. Are all metals capable of this? Not at all. According to the degree of susceptibility, three groups of substances are distinguished. For example, gold, copper and zinc will not attach to a magnet. They belong to the group of diamagnets. Magnesium, platinum and aluminum differ in weak perception. But if the composition of the mixture includes ferromagnets, then this method will be the most effective. These include, for example, iron, cobalt, nickel, terbium, holmium, thulium.

Evaporation
What method of separation of mixtures is suitable for an aqueous homogeneous solution? This is evaporation. If you only have salt water, but need clean water, don't get upset right away. You need to heat the mixture to boiling point. As a result, the water will evaporate. And at the bottom of the dish, crystals of the dissolved substance will be visible. To collect water, it must be condensed - transferred from a gaseous state to a liquid one. To do this, the vapors are cooled, touching the surface with a lower temperature, and flow into the prepared container.
Crystallization
In science, this term is considered in a broader sense. It is not just a method for obtaining pure substances. By their nature, icebergs, minerals, bones and tooth enamel are crystals.
Their growth occurs under the same conditions. Crystals are formed as a result of cooling liquids or supersaturation of steam, and in the future the temperature should no longer change. Thus, some limiting conditions are first reached. As a result, a crystallization center appears, around which atoms of a liquid, melt, gas, or glass gather.

Distillation
Surely you have heard about the water, which is called distilled. This purified liquid is necessary for the manufacture of medicines, laboratory research, and cooling systems. And they get it in special devices. They are called distillers.
Distillation is a method of separating mixtures of substances with different boiling points. Translated from Latin, the term means "draining drops." With this method, for example, alcohol and water can be separated from a solution. The first substance will begin to boil at a temperature of +78 o C. Alcohol vapors will subsequently condense. The water will remain in liquid form.
In a similar way, products of its processing are obtained from oil: gasoline, kerosene, gas oil. This process is not a chemical reaction. Oil is separated into separate fractions, each of which has its own boiling point. This happens in several stages. First, the primary separation of oil is carried out. It is cleaned from associated gas, mechanical impurities and water vapor. At the next stage, the resulting product is placed in distillation columns and heated. This is the atmospheric distillation of oil. At a temperature of less than 62 degrees, the remaining associated gas volatilizes. By heating the mixture to 180 degrees, gasoline fractions are obtained, up to 240 - kerosene, up to 350 - diesel fuel. The residue of thermal oil processing is fuel oil, which is used as a lubricant.

Chromatography
This method is named after the scientist who first used it. His name was Mikhail Semenovich Tsvet. Initially, the method was used to separate plant pigments. Literally, chromatography is translated from Greek as "I write with color." Dip the filter paper into the mixture of water and ink. The first will immediately begin to be absorbed. This is due to varying degrees of adsorbing properties. This also takes into account diffusion and the degree of solubility.
Adsorption
Some substances have the ability to attract molecules of another kind. For example, we take activated charcoal for poisoning to get rid of toxins. This process requires an interface that is between the two phases.
This method is used in the chemical industry for the separation of benzene from gaseous mixtures, purification of liquid products of oil refining, their purification from impurities.
So, in our article, we examined the main methods for separating mixtures. A person uses them both at home and on an industrial scale. The choice of method depends on the type of mixture. An important factor is the features of the physical properties of its components. To separate solutions in which individual parts are visually indistinguishable, evaporation, crystallization, chromatography and distillation methods are used. If the individual components can be determined, such mixtures are called heterogeneous. To separate them, methods of settling, filtering and using a magnet are used.