Abstract by discipline: Chemistry
On the topic: Methods for separating mixtures
Riga - 2009
Introduction…………………………………………………………………………..page 3
Types of mixtures………………………………………………………………………p.4
Methods for separating mixtures……………………………………………………..page 6
Conclusion……………………………………………………………………….page 11
List of references………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………..p.12
Introduction
In nature, substances in their pure form are very rare. Most of the objects around us are made up of a mixture of substances. In a chemical laboratory, chemists work with pure substances. If the substance contains impurities, then any chemist can separate the substance needed for the experiment from impurities. To study the properties of substances, it is necessary to purify this substance, i.e. split into component parts. The separation of a mixture is a physical process. Physical methods for the separation of substances are widely used in chemical laboratories, in the production of food products, in the production of metals and other substances.
Types of mixtures
There are no pure substances in nature. When considering boulders, granite, we are convinced that they consist of grains, veins of various colors; milk contains fats, proteins, water; oil and natural gas contain organic substances called hydrocarbons; air contains various gases; natural water is not a chemically pure substance. A mixture is a mixture of two or more dissimilar substances.
Mixtures can be divided into two large groups (ri
If the components of the mixture are visible to the naked eye, then such mixtures are called heterogeneous. For example, a mixture of wood and iron filings, a mixture of water and vegetable oil, a mixture of river sand and water, etc.
If the components of the mixture cannot be distinguished with the naked eye, then such mixtures are called homogeneous. Such mixtures as milk, oil, a solution of sugar in water, etc. are classified as homogeneous mixtures.
There are solid, liquid and gaseous substances. Substances can be mixed in any state of aggregation. The state of aggregation of a mixture determines a substance that is quantitatively superior to the rest.
Heterogeneous mixtures are formed from substances of different aggregate states, when the substances do not mutually dissolve and mix poorly (Table 1)
|
Types of heterogeneous mixtures |
|
|
before mixing |
Examples |
|
hard/solid |
Minerals; iron/sulfur |
|
solid/liquid |
Lime mortar; wastewater |
|
solid/gaseous |
Smoke; dusty air |
|
liquid/solid |
Pearl; minerals; water/ice |
|
liquid / liquid |
Milk; vegetable oil/water |
|
liquid/gaseous |
Fog; clouds |
|
gaseous/solid |
Styrofoam |
|
gaseous/liquid |
soap foam |
Homogeneous mixtures are formed when substances dissolve well in each other and mix well (Table 2).
|
Types of homogeneous mixtures |
|
|
Aggregate state of the constituent parts before mixing |
Examples |
|
hard/solid |
Alloy of gold and silver |
|
solid/liquid |
sugar/water |
|
solid/gaseous |
Vapors of iodine in the air |
|
liquid/solid |
swollen gelatin |
|
liquid / liquid |
alcohol/water |
|
liquid/gaseous |
Water/air |
|
gaseous/solid |
Hydrogen in palladium |
|
gaseous/liquid |
|
When mixtures are formed, chemical transformations usually do not occur, and the substances in the mixture retain their properties. Differences in the properties of substances are used to separate mixtures.
Methods for separating mixtures
Mixtures, both inhomogeneous and homogeneous, can be divided into constituent parts, i.e. for pure substances. Pure substances are substances that cannot be separated into two or more other substances using physical methods and do not change their physical properties. There are various methods for separating mixtures; certain methods for separating mixtures are used depending on the composition of the mixture.
- Screening;
- Filtration;
- settling;
- Decantation
- centrifugation;
- Evaporation;
- Evaporation;
- Recrystallization;
- Distillation (distillation);
- Freezing;
- The action of the magnet;
- Chromatography;
- Extraction;
- Adsorption.
Let's get acquainted with a few of them. Here it should be noted that it is easier to separate heterogeneous mixtures than homogeneous ones. Below we give examples of the separation of substances from homogeneous and heterogeneous mixtures.
Screening.
Let's imagine that granulated sugar got into the flour. Perhaps the easiest way to separate is screening. With the help of a sieve, you can easily separate small particles of flour from relatively large sugar crystals. In agriculture, screening is used to separate plant seeds from foreign debris. In construction, gravel is separated from sand in this way.
Filtration
The solid component of the suspension is separated from the liquid filtering, using paper or fabric filters, cotton wool, a thin layer of fine sand. Let's imagine that we are given a mixture of table salt, sand and clay. It is required to separate table salt from the mixture. To do this, place the mixture in a beaker with water and shake. Table salt dissolves and sand settles. Clay does not dissolve and does not settle to the bottom of the glass, so the water remains cloudy. To remove insoluble clay particles from the solution, the mixture is filtered. To do this, you need to assemble a small filter device from a glass funnel, filter paper and a tripod. The salt solution is filtered out. To do this, the filtered solution is carefully poured into a funnel with a tightly inserted filter. Sand and clay particles remain on the filter, and a clear salt solution passes through the filter. Recrystallization is used to isolate salt dissolved in water.
recrystallization, evaporation
Recrystallization a method of purification is called, in which the substance is first dissolved in water, then the solution of the substance in water is evaporated. As a result, water evaporates, and the substance is released in the form of crystals.
Let's give an example: It is required to isolate table salt from a solution.
Above, we considered an example when it was necessary to isolate table salt from a heterogeneous mixture. Now let's separate the table salt from a homogeneous mixture. The solution obtained by filtration is called the filtrate. The filtrate must be poured into a porcelain cup. Place the cup with the solution on the tripod ring and heat the solution over the flame of the spirit lamp. The water will begin to evaporate and the volume of the solution will decrease. Such a process is called evaporation. As the water evaporates, the solution becomes more concentrated. When the solution reaches a state of saturation with table salt, crystals will appear on the walls of the cup. At this point, stop heating and cool the solution. Chilled table salt will stand out in the form of crystals. If necessary, salt crystals can be separated from the solution by filtration. The solution must not be evaporated until the water has completely evaporated, since other soluble impurities can also precipitate in the form of crystals and contaminate the table salt.
Settling, decanting
Used to isolate insoluble substances from liquids. upholding. If the solid particles are large enough, they quickly settle to the bottom, and the liquid becomes transparent. It can be carefully drained from the sediment, and this simple operation also has its own name - decantation.
The smaller the solids in the liquid, the longer the mixture will settle. It is possible to separate from each other and two liquids that do not mix with each other.
centrifugation
If the particles of an inhomogeneous mixture are very small, it cannot be separated either by settling or filtering. Examples of such mixtures are milk and water-dissolved toothpaste. Such mixtures are divided centrifugation. Mixtures containing such a liquid are placed in test tubes and rotated at high speed in special apparatuses - centrifuges. As a result of centrifugation, heavier particles are "pressed" to the bottom of the vessel, and the lungs are on top. Milk is the smallest particles of fat distributed in an aqueous solution of other substances - sugars, proteins. To separate such a mixture, a special centrifuge called a separator is used. When separating milk, fats are on the surface, they are easy to separate. What remains is water with dissolved substances in it - this is skimmed milk.
Adsorption
In technology, the problem often arises of cleaning gases, such as air, from unwanted or harmful components. Many substances have one interesting property - they can "cling" to the surface of porous substances, like iron to a magnet. Adsorption called the ability of some solids to absorb gaseous or dissolved substances on their surface. Substances capable of adsorption are called adsorbents. Adsorbents are solid substances in which there are many internal channels, voids, pores, i.e. they have a very large total absorbing surface. Adsorbents are activated carbon, silica gel (in the box with new shoes you can find a small bag of white peas - this is silica gel), filter paper. Different substances "attach" to the surface of adsorbents differently: some are held on the surface firmly, others are weaker. Activated carbon is able to absorb not only gaseous, but also substances dissolved in liquids. In case of poisoning, it is taken so that toxic substances are adsorbed on it.
Distillation (distillation)
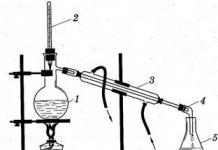
Two liquids that form a homogeneous mixture, such as ethyl alcohol and water, are separated by distillation or distillation. This method is based on the fact that the liquid is heated to the boiling point and its vapor is removed through a gas outlet tube into another vessel. Cooling, the vapor condenses, and impurities remain in the distillation flask. The distillation apparatus is shown in Fig. 2

The liquid is placed in a Wurtz flask (1), the neck of the Wurtz flask is tightly closed with a stopper with a thermometer inserted into it (2), while the mercury reservoir should be at the level of the outlet tube opening. The end of the outlet tube is inserted through a tightly fitted stopper into the Liebig refrigerator (3), at the other end of which the allonge (4) is fixed. The narrowed end of the allonge is lowered into the receiver (5). The lower end of the refrigerator jacket is connected with a rubber hose to a water tap, and from the upper end a drain is made into the sink. The refrigerator jacket should always be filled with water. The Wurtz flask and condenser are fixed in separate racks. The liquid is poured into the flask through a funnel with a long tube, filling the distillation flask to 2/3 of its volume. For uniform boiling, several boiling points are placed on the bottom of the flask - glass capillaries sealed at one end. After closing the flask, water is supplied to the refrigerator and the liquid in the flask is heated. Heating can be carried out on a gas burner, electric stove, water, sand or oil bath - depending on the boiling point of the liquid. In no case should flammable and combustible liquids (alcohol, ether, acetone, etc.) be heated over an open fire in order to avoid accidents: only a water or other bath should be used. The liquid should not be evaporated completely: 10-15% of the initially taken volume should remain in the flask. A new portion of the liquid can be poured only when the flask has cooled slightly.
Freezing
Substances with different melting points are separated by the method freezing, cooling the solution. By freezing, you can get very clean water at home. To do this, pour tap water into a jar or mug and put it in the freezer of the refrigerator (or take it out in the cold in winter). As soon as about half of the water turns into ice, the unfrozen part of it, where impurities accumulate, must be poured out, and the ice allowed to melt.
In industry and in laboratory conditions, methods for separating mixtures are used, based on other different properties of the constituent parts of the mixture. For example, iron filings can be isolated from a mixture magnet. The ability of substances to dissolve in various solvents is used in extraction- a method for separating solid or liquid mixtures by treating them with various solvents. For example, iodine from an aqueous solution can be isolated by any organic solvent in which iodine dissolves better.
Conclusion
In laboratory practice and in everyday life, it is very often necessary to isolate individual components from a mixture of substances. Note that mixtures include two or more substances, divided into two large groups: homogeneous and heterogeneous. There are various ways of separating mixtures, such as filtration, evaporation, distillation (distillation) and others. Methods for separating mixtures mainly depend on the type and composition of the mixture.
List of used literature
1. S.Ozols, E.Lepiņš chemistry for elementary school., 1996. P. 289
2. Information from the Internet
The material of the lesson contains information about various ways of separating mixtures and purifying substances. You will learn how to use knowledge of the differences in the properties of the components of a mixture to select the optimal method for separating a given mixture.
Topic: Initial chemical ideas
Lesson: Methods for separating mixtures and purifying substances
Let us define the difference between "methods for separating mixtures" and "methods for purifying substances." In the first case, it is important to obtain in pure form all the components that make up the mixture. When purifying a substance, obtaining impurities in a pure form is usually neglected.
SETTLEMENT
How to separate a mixture of sand and clay? This is one of the stages in ceramic production (for example, in the production of bricks). To separate such a mixture, the settling method is used. The mixture is placed in water and stirred. Clay and sand settle in water at different rates. Therefore, sand will settle much faster than clay (Fig. 1).
Rice. 1. Separation of a mixture of clay and sand by settling
The settling method is also used to separate mixtures of water-insoluble solids with different densities. For example, a mixture of iron and sawdust can be separated in this way (the sawdust will float in water, while the iron will settle).
A mixture of vegetable oil and water can also be separated by settling, because the oil does not dissolve in water and has a lower density (Fig. 2). Thus, by settling, it is possible to separate mixtures of liquids insoluble in each other with different densities.

Rice. 2. Separation of a mixture of vegetable oil and water by settling
To separate a mixture of table salt and river sand, you can use the settling method (when mixed with water, the salt will dissolve, the sand will settle), but it will be more reliable to separate the sand from the salt solution by another method - the filtration method.
Filtration of this mixture can be carried out using a paper filter and a funnel lowered into a glass. Grains of sand remain on the filter paper, and a clear solution of table salt passes through the filter. In this case, the river sand is the sediment, and the salt solution is the leachate (Fig. 3).

Rice. 3. Using filtration method to separate river sand from salt solution
Filtration can be carried out not only with filter paper, but also with other porous or loose materials. For example, bulk materials include quartz sand, and porous materials include glass wool and baked clay.
Some mixtures can be separated using the "hot filtration" method. For example, a mixture of sulfur and iron powders. Iron melts at over 1500 C and sulfur around 120 C. Molten sulfur can be separated from the iron powder using heated glass wool.
Salt can be isolated from the filtrate by evaporation, i.e. heat the mixture and the water will evaporate and the salt will remain on the porcelain cup. Evaporation, the partial evaporation of water, is sometimes used. As a result, a more concentrated solution is formed, upon cooling of which the solute is released in the form of crystals.
If a substance capable of magnetization is present in the mixture, then it is easy to isolate it in its pure form using a magnet. For example, a mixture of sulfur and iron powders can be separated in this way.
The same mixture can be separated by another method, using knowledge of the wettability of the components of the mixture with water. Iron is wetted by water, i.e. water spreads over the surface of the iron. Sulfur is not wetted by water. If you put a piece of sulfur in water, it will sink, because. The density of sulfur is greater than the density of water. But the sulfur powder will emerge, because. air bubbles stick to the grains of sulfur that are not wetted by water and push them to the surface. To separate the mixture, you need to place it in water. The sulfur powder will float and the iron will sink (Fig. 4).

Rice. 4. Separation of a mixture of sulfur and iron powders by flotation
The method of separating mixtures based on the difference in the wettability of the components is called flotation (French flotter - to float). Consider a few more methods for the separation and purification of substances.
One of the oldest methods of separating mixtures is distillation (or distillation). Using this method, it is possible to separate components that are soluble in each other and have different boiling points. This is how distilled water is obtained. Water with impurities is boiled in one vessel. The resulting water vapor condenses upon cooling in another vessel in the form of already distilled (pure) water.

Rice. 5. Obtaining distilled water
Components with similar properties can be separated using the chromatography method. This method is based on the different absorption of the substances to be separated by the surface of another substance.
For example, red ink can be separated into its components (water and dye) by chromatography.

Rice. 6. Separation of red ink by paper chromatography
In chemical laboratories, chromatography is carried out using special instruments - chromatographs, the main parts of which are a chromatographic column and a detector.
Adsorption is widely used in chemistry to purify certain substances. It is the accumulation of one substance on the surface of another substance. Adsorbents include, for example, activated carbon.
Try dropping an activated charcoal tablet into a container of colored water, stir, filter, and you will see that the filtrate has become colorless. Carbon atoms attract molecules, in this case, the dye.
Currently, adsorption is widely used for water and air purification. For example, water filters contain activated carbon as an adsorbent.
1. Collection of tasks and exercises in chemistry: 8th grade: to the textbook by P.A. Orzhekovsky and others. "Chemistry, Grade 8" / P.A. Orzhekovsky, N.A. Titov, F.F. Hegel. – M.: AST: Astrel, 2006.
2. Ushakova O.V. Chemistry workbook: 8th grade: to the textbook by P.A. Orzhekovsky and others. “Chemistry. Grade 8” / O.V. Ushakova, P.I. Bespalov, P.A. Orzhekovsky; under. ed. prof. P.A. Orzhekovsky - M .: AST: Astrel: Profizdat, 2006. (p. 10-11)
3. Chemistry: 8th grade: textbook. for general institutions / P.A. Orzhekovsky, L.M. Meshcheryakova, L.S. Pontak. M.: AST: Astrel, 2005.(§4)
4. Chemistry: inorg. chemistry: textbook. for 8 cells. general institutions / G.E. Rudzitis, FuGyu Feldman. - M .: Education, JSC "Moscow textbooks", 2009. (§ 2)
5. Encyclopedia for children. Volume 17. Chemistry / Chapter. edited by V.A. Volodin, leading. scientific ed. I. Leenson. – M.: Avanta+, 2003.
Additional web resources
1. A single collection of digital educational resources ().
2. Electronic version of the journal "Chemistry and Life" ().
Homework
From the textbook P.A. Orzhekovsky and others. "Chemistry, Grade 8" With. 33 Nos. 2,4,6,T.
pure substancecontains particles only one kind. Examples are silver (containing only silver atoms), sulfuric acid, and carbon monoxide ( IV) (contain only the molecules of the corresponding substances). All pure substances have constant physical properties, for example, melting point (T pl ) and boiling point ( T bale ).
A substance is not pure if it contains any amount of one or more other substances -impurities.
Contaminants lower the freezing point and raise the boiling point of a clean liquid. For example, if salt is added to water, the freezing point of the solution will decrease.
Mixes consist of two or more substances. Soil, sea water, air are all examples of different mixtures. Many mixtures can be separated into their component parts − Components - based on the difference in their physical properties.
Traditional methods that are used in laboratory practice to separate mixtures into individual components are:
filtration,
settling followed by decantation,
separation using a separating funnel,
centrifugation,
evaporation,
crystallization,
distillation (including fractional distillation),
chromatography,
sublimation and others.
Filtration. Filtration is used to separate liquids from fine solid particles suspended in it.(fig.37) , i.e. filtering liquid through finely porous materials -filters, which allow liquid to pass through and retain solid particles on their surface. The liquid that has passed through the filter and freed from the solid impurities in it is called filtrate.
Often used in laboratory practicesmooth and folded paper filters(fig.38) made from non-glued filter paper.
To filter hot solutions (for example, for the purpose of recrystallization of salts), a specialhot filter funnel(fig.39) with electric or water heating).
Often usedvacuum filtration. Vacuum filtration is used to speed up filtration and more completely free the precipitate from solution. For this purpose, a vacuum filtration device is assembled. (fig.40) . It consists ofBunsen flask, Buchner porcelain funnel, safety bottle and vacuum pump(usually water jet).
In the case of filtering a suspension of a sparingly soluble salt, the crystals of the latter can be washed with distilled water on a Buchner funnel to remove the initial solution from their surface. For this purpose, use washer(fig.41) .
Decantation. Liquids can be separated from insoluble solidsdecantation(fig.42) . This method can be used if the solid has a higher density than the liquid. For example, if river sand is added to a glass of water, then when settling, it will settle to the bottom of the glass, because the density of sand is greater than that of water. Then the water can be separated from the sand by simply draining. This method of settling and subsequent draining of the filtrate is called decantation.
Centrifugation.D To accelerate the process of separating very small particles that form stable suspensions or emulsions in a liquid, the method is used. centrifugation. This method can be used to separate mixtures of liquid and solid substances that differ in density. The division is carried out in manual or electric centrifuges(fig.43) .
Separation of two immiscible liquids, having different densities and not forming stable emulsions,can be done with a separating funnel (fig.44) . So you can separate, for example, a mixture of benzene and water. Benzene layer (density = 0.879 g/cm 3 ) located above a layer of water, which has a high density ( = 1.0 g/cm 3 ). By opening the stopcock of the separating funnel, you can carefully drain the bottom layer and separate one liquid from another.
Evaporation(fig.45) - this method involves the removal of a solvent, such as water, from a solution by heating it in an evaporating porcelain dish. In this case, the evaporated liquid is removed, and the dissolved substance remains in the evaporating dish.
Crystallization- this is the process of separating crystals of a solid when a solution is cooled, for example, after it has been evaporated. It should be borne in mind that large crystals are formed when the solution is slowly cooled. Upon rapid cooling (eg cooling under running water), fine crystals form.
Distillation- a method of cleaning a substance based on the evaporation of a liquid when heated, followed by condensation of the resulting vapors. Purification of water from salts (or other substances, for example, dyes) dissolved in it by distillation is called distillation, and the purified water itself is distilled.
Fractional distillation(fig.46) used to separate mixtures of liquids with different boiling points. A liquid with a lower boiling point boils faster and passes through fractional column(ordephlegmator). When this liquid reaches the top of the fractionation column, it enters thefridge, cooled by water andallongegoing toreceiver(flask or test tube).
Fractional distillation can separate, for example, a mixture of ethanol and water. Boiling point of ethanol 78 0 C, and water 100 0 C. Ethanol evaporates more easily and enters the receiver through the condenser first.
Sublimation - This method is used to purify substances capable of changing from a solid state to a gaseous state when heated, bypassing the liquid state. Further, the vapors of the substance to be purified are condensed, and impurities that are not able to sublimate are separated.
Methods for separating mixtures
Most of the substances on our planet are not in their pure form, but in compounds and mixtures, along with other substances.
So, the composition of granite includes three substances that are visible to the naked eye.
But milk seems homogeneous to us until it turns sour. Sour
milk separates into a clear whey and a white solid precipitate - protein
casein. Man long ago uses these substances , included in milk, highlighting them
from the mixture. Curd is prepared from insoluble protein - casein, and soluble
whey proteins are used for clinical nutrition.
How can mixtures be separated?
1. If the substance is insoluble in water, such as cereals (rice, buckwheat, semolina, etc.), river sand, chalk, clay, then you can use the filtration method.
Filtration-filtering liquids (gases) through a filter in order to purify them from solid impurities.

1. Putting up a filter. We place it in a funnel, slightly wetting it with water.
2. Insert the funnel with the filter into the flask.
3. Pass a mixture of undissolved matter and water through the filter.
Conclusion. Water purified by filtration freely passed through the filter; a substance insoluble in water remains on the filter.
2. If the solid is soluble in water (salt, sugar, citric acid), then to separatemixtures, the evaporation method can be used.
Evaporation- separation of solids dissolved in a liquid by converting it into vapor.
In a glass of water, the salt did not disappear, although it became invisible - the solution is transparent. Evaporation made it possible to isolate a substance dissolved in water from a mixture of substances (water and salt). Salt crystals are visible on the glass. This confirms the conclusion that that each substance (both water and salt) of the mixture retains its properties.
Conclusion. Soluble substances can be isolated from a solution.
3 .To separate liquids soluble in each other, to obtain pure (without impurities) water, the distillation method is used
(or distillation)
Distillation-distillation, separation of substances contained in liquid mixtures according to boiling points, followed by cooling of the vapor.
In nature, water in its pure form (without salts) does not occur. Oceanic, sea, river, well and spring water are varieties of salt solutions in water. However, often people need clean water that does not contain salts (used in car engines; in chemical production to obtain various solutions and substances; in the manufacture of photographs). Such water is called distilled, and the method of obtaining it is called distillation.

We heat tap water over the flame of an alcohol lamp in a test tube, closed with a cork with a gas outlet tube. We lower the end of the tube into a clean, dry test tube placed in a glass with ice. Drops of distilled (purified from salts and impurities) water will appear on the bottom and walls of a test tube in a glass with ice.
Exercise
1. Look into an empty kettle in which water is boiled. Are there white deposits (scale) on the walls and bottom of substances that have been dissolved in water?
2. Droplets of water flow from the lid of the kettle in which water is boiled. Which water - on the lid or in the kettle itself - contains more salts? Explain your answer.
3. What is the name of the process shown in the picture?

4. If the mixture contains iron, then a magnet can be used to isolate it, because. iron and its alloys are attracted by a magnet.
5. To separate two immiscible liquids (oil and water, sunflower oil and water), you need to use a separating funnel.

The liquid with a higher density will merge into a glass, and a lighter liquid will remain in the separating funnel.
Every substance contains impurities. A substance is considered pure if it contains almost no impurities.
Mixtures of substances are either homogeneous or heterogeneous. In a homogeneous mixture, the components cannot be detected by observation, but in an inhomogeneous mixture it is possible.
Some physical properties of a homogeneous mixture differ from those of the components.
In a heterogeneous mixture, the properties of the components are preserved.
Heterogeneous mixtures of substances are separated by settling, filtering, sometimes by the action of a magnet, and homogeneous mixtures are separated by evaporation and distillation (distillation).
Pure substances and mixtures
We live among chemicals. We inhale air, and this is a mixture of gases (nitrogen, oxygen and others), we exhale carbon dioxide. We wash ourselves with water - this is another substance, the most common on Earth. We drink milk - a mixture of water with the smallest droplets of milk fat, and not only: there is also milk protein casein, mineral salts, vitamins and even sugar, but not the one with which they drink tea, but a special milk - lactose. We eat apples, which consist of a whole range of chemicals - sugar, malic acid, vitamins... apple, but also any other food. We not only live among chemicals, but we ourselves are made of them. Every person - his skin, muscles, blood, teeth, bones, hair are built of chemicals, like a house of bricks. Nitrogen, oxygen, sugar, vitamins are substances of natural, natural origin. Glass, rubber, steel are also substances, more precisely, materials (mixtures of substances). Both glass and rubber are of artificial origin; they did not exist in nature. Completely pure substances are not found in nature or are very rare.
Each substance always contains a certain amount of impurities. A substance that contains almost no impurities is called pure. They work with such substances in a scientific laboratory, a school chemistry room. Note that absolutely pure substances do not exist.
An individual pure substance has a certain set of characteristic properties (constant physical properties). Only pure distilled water has tmelt = 0 °С, tboil = 100 °С, and has no taste. Sea water freezes at a lower temperature, and boils at a higher temperature, its taste is bitter-salty. The water of the Black Sea freezes at a lower temperature and boils at a higher temperature than the water of the Baltic Sea. Why? The fact is that sea water contains other substances, for example, dissolved salts, i.e. it is a mixture of various substances, the composition of which varies over a wide range, but the properties of the mixture are not constant. The concept of "mixture" was defined in the 17th century. English scientist Robert Boyle: "A mixture is an integral system consisting of heterogeneous components."
Almost all natural substances, food products (except salt, sugar, and some others), many medicinal and cosmetic products, household chemicals, and building materials are mixtures.
Comparative characteristics of a mixture and a pure substance
Each substance contained in a mixture is called a component.
Classification of mixtures
There are homogeneous and heterogeneous mixtures.
Homogeneous mixtures (homogeneous)
Add a small portion of sugar to a glass of water and stir until all the sugar is dissolved. The liquid will taste sweet. Thus, the sugar did not disappear, but remained in the mixture. Ho, we will not see its crystals, even when examining a drop of liquid in a powerful microscope. The prepared mixture of sugar and water is homogeneous; the smallest particles of these substances are evenly mixed in it.
Mixtures in which components cannot be detected by observation are called homogeneous.
Most metal alloys are also homogeneous mixtures. For example, an alloy of gold and copper (used to make jewelry) lacks red copper particles and yellow gold particles.
From materials that are homogeneous mixtures of substances, many items for various purposes are made.
All mixtures of gases, including air, belong to homogeneous mixtures. There are many homogeneous mixtures of liquids.
Homogeneous mixtures are also called solutions, even if they are solid or gaseous.
Let us give examples of solutions (air in a flask, table salt + water, small change: aluminum + copper or nickel + copper).
Heterogeneous mixtures (heterogeneous)
You know that chalk does not dissolve in water. If its powder is poured into a glass of water, then chalk particles can always be found in the resulting mixture, which are visible to the naked eye or through a microscope.
Mixtures in which components can be detected by observation are called heterogeneous.
Heterogeneous mixtures include most minerals, soil, building materials, living tissues, turbid water, milk and other foods, some drugs and cosmetics.
In a heterogeneous mixture, the physical properties of the components are preserved. So, iron filings mixed with copper or aluminum do not lose their ability to be attracted to a magnet.
Some types of heterogeneous mixtures have special names: foam (for example, foam, soap suds), suspension (a mixture of water with a small amount of flour), emulsion (milk, well-shaken vegetable oil with water), aerosol (smoke, fog).
Methods for separating mixtures
In nature, substances exist in the form of mixtures. For laboratory research, industrial production, for the needs of pharmacology and medicine, pure substances are needed.
There are many methods for separating mixtures. They are chosen taking into account the type of mixture, state of aggregation and differences in the physical properties of the components.
Methods for separating mixtures

These methods are based on differences in the physical properties of the components of the mixture.
Consider methods for separating heterogeneous and homogeneous mixtures.
Blend example |
Separation method |
Suspension - a mixture of river sand with water |
settling Separation by settling is based on different densities of the substances. Heavier sand settles to the bottom. You can also separate the emulsion: to separate oil or vegetable oil from water. In the laboratory, this can be done using a separating funnel. Oil or vegetable oil forms the top, lighter layer. As a result of settling, dew falls out of the fog, soot is deposited from smoke, cream is settled in milk. |
A mixture of sand and table salt in water |
Filtration The separation of heterogeneous mixtures by filtration is based on the different solubility of substances in water and on different particle sizes. Only particles of substances commensurate with them pass through the pores of the filter, while larger particles are retained on the filter. So you can separate a heterogeneous mixture of table salt and river sand. Various porous substances can be used as filters: cotton wool, coal, fired clay, pressed glass, and others. The filtering method is the basis for the operation of household appliances, such as vacuum cleaners. It is used by surgeons - gauze bandages; drillers and workers of elevators - respiratory masks. With the help of a tea strainer for filtering tea leaves, Ostap Bender - the hero of the work of Ilf and Petrov - managed to take one of the chairs from Ellochka Ogre ("The Twelve Chairs"). |
A mixture of iron powder and sulfur |
Action by magnet or water Iron powder was attracted by a magnet, but sulfur powder was not. The non-wettable sulfur powder floated to the surface of the water, while the heavy wettable iron powder settled to the bottom. |
A solution of salt in water is a homogeneous mixture |
Evaporation or crystallization The water evaporates and salt crystals remain in the porcelain cup. When water is evaporated from lakes Elton and Baskunchak, table salt is obtained. This separation method is based on the difference in the boiling points of the solvent and the solute. If a substance, such as sugar, decomposes when heated, then the water is not completely evaporated - the solution is evaporated, and then sugar crystals are precipitated from a saturated solution. Sometimes it is required to remove impurities from solvents with a lower boiling point, for example, water from salt. In this case, the vapors of the substance must be collected and then condensed upon cooling. This method of separating a homogeneous mixture is called distillation, or distillation. In special devices - distillers, distilled water is obtained, which is used for the needs of pharmacology, laboratories, and car cooling systems. At home, you can design such a distiller. If, however, a mixture of alcohol and water is separated, then the first to be distilled off (collected in a receiving test tube) is alcohol with tboil = 78 °C, and water will remain in the test tube. Distillation is used to obtain gasoline, kerosene, gas oil from oil. |
Chromatography is a special method for separating components based on their different absorption by a certain substance.
If you hang a strip of filter paper over a vessel with red ink, immersing only the end of the strip in them. The solution is absorbed by the paper and rises along it. But the border of the rise of the paint lags behind the border of the rise of the water. This is how the separation of two substances occurs: water and the coloring matter in the ink.
With the help of chromatography, the Russian botanist M. S. Tsvet was the first to isolate chlorophyll from the green parts of plants. In industry and laboratories, instead of filter paper for chromatography, starch, coal, limestone, and aluminum oxide are used. Are substances always required with the same degree of purification?
For different purposes, substances with different degrees of purification are needed. Cooking water is sufficiently settled to remove impurities and chlorine used to disinfect it. Drinking water must first be boiled. And in chemical laboratories for the preparation of solutions and experiments, in medicine, distilled water is needed, as purified as possible from the substances dissolved in it. Highly pure substances, the content of impurities in which does not exceed one millionth of a percent, are used in electronics, semiconductor, nuclear technology and other precision industries.