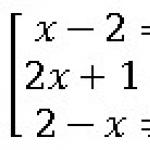1) Physical methods: evaporation (evaporation), distillation
Evaporation – incomplete evaporation of the solvent (volume reduction – concentration)
Evaporation – evaporation of the solvent to dryness (followed by dissolution of the dry residue in a small volume)
Distillation – separation of volatile components
2) Chemical methods: precipitation, coprecipitation
Precipitation – separation (systematic course of analysis); concentration (precipitation of the ion to be determined from a large volume of the analyzed solution and dissolution of the precipitate in a small volume)
Co-precipitation – simultaneous precipitation from the same solution of a microcomponent soluble under given conditions with a precipitated macrocomponent.
Causes of coprecipitation: 1) surface adsorption - the co-precipitated substance is adsorbed on the surface of the collector and deposited with it; 2) occlusion - mechanical capture of part of the mother solution with the coprecipitated ion inside the collector sediment; 3) inclusion – formation of mixed crystals
Co-precipitation is used to concentrate substances present in microquantities in the analyzed solution, followed by their determination in the concentrate.
3) Physico-chemical methods: extraction, chromatography
Extraction – a method of extracting a substance from a solution or dry mixture using a suitable solvent. To extract from a solution, solvents are used that are immiscible with this solution, but in which the substance dissolves better than in the first solvent. Extraction is used in the chemical, oil refining, food, metallurgical, and pharmaceutical industries.
Chromatography – dynamic sorption method for separating and analyzing mixtures of substances, as well as studying the physicochemical properties of substances. It is based on the distribution of substances between two phases - stationary (solid phase or liquid bound on an inert carrier) and mobile (gas or liquid phase).
88. Methods of qualitative chemical analysis
Microcrystalloscopic analysis
Reactions that produce compounds with characteristic crystal shapes can be used to detect cations and anions. The shape and rate of crystal formation are affected by the reaction conditions. A significant role in microcrystalloscopic reactions is played by the rapid evaporation of the solvent, which leads to concentration of the solution and, consequently, an increase in the sensitivity of ion determination.
Pyrochemical analysis
When heating substances in a burner flame, various characteristic phenomena can be observed: evaporation, melting, color change, coloring of the flame. All these phenomena are used in qualitative analysis for preliminary testing of a substance. Sometimes, with the help of pyrochemical reactions, it is possible to increase selectivity and sensitivity of determination. Pyrochemical reactions used for mineral analysis in the field.
Flame coloring
When a metal salt solution is introduced into a flame, a number of complex processes occur: evaporation, formation of solid aerosols, dissociation, ionization, interaction with oxygen, excitation of atoms, ions and molecules. The end result of these processes is the analytically used effect - flame glow.
89. Methods for determining the quantitative composition of compounds

90. Basic physical quantities
Physical quantity – a physical property of a material object, physical phenomenon, process that can be characterized quantitatively.
Physical quantity value – a number characterizing this physical quantity, indicating the unit of measurement on the basis of which they were obtained.
System of physical units – a set of units of measurement of physical quantities, in which there is a certain number of so-called basic units of measurement, and the remaining units of measurement can be expressed through these basic units. SI (System International) – international system of units. SI is the most widely used system of units in the world, both in everyday life and in science and technology.
In the SI system, each basic quantity has a corresponding unit: unit of length– meter (m); unit of time– second (s); unit of mass– kilogram (kg); units electric current intensity– ampere (A); temperature unit– kelvin (K); unit of quantity of substance– mole (mol); unit of luminous intensity– candela (cd)
In practical use, International System units often turn out to be either too large or too small, so decimal multiples and submultiples can be formed using special prefixes.
| soundboard | Yes | 10 1 | deci | d | 10 -1 |
| hecto | G | 10 2 | centi | With | 10 -2 |
| kilo | To | 10 3 | Milli | m | 10 -3 |
| mega | M | 10 6 | micro | mk | 10 -6 |
| giga | G | 10 9 | nano | n | 10 -9 |
| tera | T | 10 12 | pico | P | 10 -12 |
| peta | P | 10 15 | femto | F | 10 -15 |
| exa | E | 10 18 | atto | A | 10 -18 |
91. The concept of physical methods and their classification
92. Use of physical methods in expert research
93. The concept of the physical quantity “density”. Methods for determining density
Density – a physical quantity equal to the ratio of body mass to its volume ( ρ = m/V). Based on the definition of density, its dimension kg/m 3 in the SI system.
The density of a substance depends on the mass of the atoms of which it consists, and on the packing density of atoms and molecules in the substance. The greater the mass of atoms and the closer they are located to each other, the greater the density.
Density meters used to measure the density of liquids, gases and solids.
Density of inhomogeneous matter - the ratio of mass and volume when the latter contracts to the point at which density is measured. The ratio of the densities of two substances under certain standard physical conditions is called relative density; for liquid and solid substances it is measured at temperature t, usually relative to the density of distilled water at 4°C, for gases– in relation to the density of dry air or hydrogen under normal conditions ( T= 273K, p = 1.01 10 5 Pa).
For bulk and porous solids, densities are distinguished true (the mass of a unit volume of a dense material that does not contain pores), apparent (the mass of a unit volume of porous material made from grains or granules) and bulk (the mass of a unit volume of a layer of material).
94. The concept of the physical quantity “mass”. Methods for determining mass
Weight – a scalar physical quantity, one of the main characteristics of matter, determining its inertial and gravitational properties. There are inertial mass and gravitational mass.
The concept of mass was introduced into mechanics I. Newton. In classical mechanics Newton mass is included in the definition of momentum (quantity of movement) of a body: impulse R proportional to the speed of the body V , p=mv (1). Proportionality factor – a constant value for a given body m– and there is body mass. The equivalent definition of mass is obtained from the equation of motion of classical mechanics F=ma(2). Here mass is the coefficient of proportionality between the force acting on the body F and the acceleration of the body caused by it a. The mass determined by relations (1) and (2) is called inertial (inertial) mass ; it characterizes the dynamic properties of the body, is a measure of the inertia of the body: with a constant force, the greater the mass of the body, the less acceleration it acquires, i.e. the slower the state of its motion changes.
In the theory of gravity Newton mass acts as a source of the gravitational field. Each body creates a gravitational field proportional to the mass of the body (and is affected by the gravitational field created by other bodies, the strength of which is also proportional to the mass of the bodies). This field causes the attraction of any other body to this body with a force determined by Newton’s law of gravity: F = G* (m 1 *m 2 / R 2) - (3), where R– distance between bodies, G is the universal gravitational constant, a m 1 And m 2– masses of attracting bodies.
From formula (3) it is easy to obtain the formula for weight R body mass m in the Earth's gravitational field: P = mg(4). Here g = G*M/r 2- acceleration of gravity in the Earth's gravitational field. The mass determined by relations (3) and (4) is called gravitational mass of the body .
Scales - a device for determining the mass of bodies (weighing) by the weight acting on them, approximately considering it equal to the force of gravity. Let us consider as an example the measurement of body weight, which we measure using ordinary equal-arm scales. Under the influence of gravity, forces are created. The mass of the body, together with these forces, presses on one cup, and the mass of the weights on the other. By selecting weights, we achieve balance, i.e. equality of these forces. This gives us the right to say that the mass of the body being weighed is equal to the mass of the weights, assuming that the force of gravity at the distance between the cups remains the same. As you can see, to measure mass we had to convert the masses of the body and weights into forces, and to compare forces with each other, convert their action into mechanical movement of the levers of the scales.
Course work:
Methods of separation and concentration in elemental analysis
Introduction
General characteristics of separation methods
Extraction as a separation method
General characteristics of concentration methods
Co-precipitation as a concentration method
Conclusion
Bibliography
Introduction
The development of analytical chemistry proceeds in two main ways: the development of the most selective methods for the determination of individual substances and the optimal combination of separation and concentration methods with non-selective methods of determination in combined methods of analysis. In this case, the selectivity of the method means the ability to register an analytical signal corresponding to the substance being determined against the background created by accompanying impurities and the matrix. The concept of combined is in full accordance with the semantic content of this word: connected together to achieve a common goal. Accordingly, there may be combined separation methods, which aim to improve the separation, and combined analytical methods, which provide an optimal combination of preliminary separation with the final determination. The widespread use of combined methods of analysis, primarily chromatographic, cannot be considered only as a consequence of the limited selectivity of the known methods for the direct determination of substances in the object of analysis.
In addition to the important role of separation and concentration methods for combined methods of analysis, separation methods have their own value in solving preparative problems. Analysts constantly need high-purity substances: solvents, primarily water, reagents, and finally all the substances that they analyze. The tasks of preparing standard samples are as varied as the objects of analysis. And it is not always possible to use ready-made samples and their components of the required degree of purity. In its preparative interests, analytical chemistry comes into close contact with chemical technology. Methods of separation and concentration developed by analysts are often implemented in technological processes without undergoing fundamental changes. In this case, we can talk about both large-scale extraction technologies for the production of rare metals, and about processes in the pharmaceutical industry, in biochemical production, where the line between the scale of laboratory experiment and industrial production is practically absent.
General characteristics of separation methods
By separation methods we mean a set of chemical and physical processes characteristic of them and methods for their implementation. The process itself, for example, of separating substances between two liquid phases, is not yet a separation method. In combination with a method of implementation that ensures the transition of substances from one phase to another as a result of their equilibrium distribution between phases, such a process will become extraction, and in combination with a chromatographic method - liquid-liquid chromatography.
Difficulties in any attempt to systematize separation methods are introduced by combined methods of analysis. The names “gas” and “liquid” chromatography hide both methods for the chromatographic separation of substances in the gas and liquid phases, and corresponding combined methods.
There is still no generally accepted classification of separation and concentration honeys. When considering various methods together, you most often encounter a simple listing of them. Or with grouping according to some formal criteria outside the general classification. When systematizing separation methods, in the simplest case, the starting point is that the method belongs to one or another field of science that gave birth to it: chemical, physicochemical, physical methods. Let us classify separation methods in the tables below.
Table 1. Separation methods based on the formation of a new phase by the isolated substance
Aggregate state of the phase in which the initial mixture of substances is located Aggregate state of the isolated phase Solid body Gas Liquid Liquid Precipitation, electrodeposition, freezing, crystallization Distillation, distillation, rectification - Gas - Freezing Solid - High temperature distillation when interacting with a gaseous reagent, sublimation Selective dissolution
Phase separation methods comprise four groups and are based on:
2.differences in the distribution of substances between phases; .differences in mass transfer, manifested during the induced transition of a substance from one phase to another through the third phase separating them; .mechanisms of intraphase separation. For the first group of methods, the characteristic features are the aggregative states of the initial mixture of substances and the isolated phase (Table 1). Methods of the second group are based on the general patterns of distribution of substances between phases and can be characterized by their state of aggregation and the method of carrying out the process of interphase distribution. For the third and fourth groups, in addition to the state of aggregation of the phases, a characteristic feature is the nature of the driving forces of the process. The significance for analytical chemistry of the separation methods included in the first group is far from ambiguous. The processes of freezing out both liquid and gas phases and selective dissolution of the solid phase are used relatively rarely. Freezing is used in gas analysis to separate moisture and to cryogenically concentrate higher-boiling impurities. Selective dissolution is used in two variants: partial or complete dissolution of the matrix and selective dissolution of phases. An example of complete dissolution of the matrix is the dissolution of steels and alloys when determining non-metallic inclusions: oxides, carbides, nitrides. Of utmost importance for analytical chemistry are separation methods based on differences in the distribution of substances between phases: extraction, sorption, various chromatographic methods. A characteristic feature of the separation methods of this group, in addition to the phase system, is the method of carrying out the process of interphase separation (Table 2) Table 2. Separation methods based on differences in the distribution of substances between phases Phase system Method of implementing the interphase separation process Single equilibrium distribution Multiple repetitions of the distribution process change, adsorption, gel permeation, affinity chromatography Liquid- gasGas extractionBubblingGas-liquid and liquid-gas chromatographyCritical substance-solid (liquidity)Supercritical fluid extractionMultistage fluid extractionSupercritical fluid chromatography
Extraction as a separation method
Of the separation methods based on a single equilibrium separation of substances, extraction has the greatest practical importance. Extraction is one of the most reliable, very effective and widespread methods for concentrating and separating substances. The research and application of extraction is a leading, rapidly developing direction in modern chemistry. Extraction refers to both the process of distributing substances between two phases and the separation method based on this process. In the most general case, we can consider phase equilibria in liquid-liquid, liquid-gas systems. A wide variety of liquid phases are possible: water and aqueous solutions, organic solvents and solutions of other organic compounds in them, molten salts and metals, melts of solid organic compounds under normal conditions. The gas extraction method (liquid-gas system and, less commonly, solid-gas system) has a narrower purpose - for the analysis of gaseous and volatile compounds in condensed phases, and differs from conventional extraction only in that gas is used as an extractant, which does not interfere with the analytical determination of gaseous compounds. impurities. Many methods close to extraction, such as paper and column partition chromatography, are based on the distribution of substances between two liquid phases. In partition chromatography, one of the phases, organic or aqueous, is fixed on an inert carrier, while the other is moving. This achieves multiple exchanges between phases. Of particular importance is the extraction of various metal compounds from aqueous solutions into organic solvents that are immiscible with them. Extraction is carried out by 1) bringing solutions (metal ions and extractant) into contact (mixing); 2) mechanical phase separation; 3) regeneration of the extractant. The areas of application of extraction are very diverse. Extraction makes it possible to separate even small amounts of impurities from the base, which is especially important when obtaining and analyzing high-purity materials, separating radioisotopes, purifying biological materials, etc. Extraction is used less frequently to separate the base from traces and, as a rule, only in cases where impurities cannot be isolated. Macrocomponents are usually extracted in the form of complex metal halide acids (for example, iron from HCI solutions with diethyl ether) or coordination-solvation salts. Microimpurities are often extracted in the form of intracomplex compounds, less often - in the form of complex metal acids. Extraction is also effective in separating components with similar properties, including high-boiling substances and azeotropic mixtures. Extraction is widely used to increase the sensitivity of determinations by many chemical and physicochemical methods of analysis. Extraction plays a significant role in the study of equilibria in solutions, complex formation processes, and in general in the study of the state of substances in solutions. Such advantages of extraction as versatility, rapidity, ease of implementation, speed, low operating temperatures, accessibility, lack of complex equipment, relatively small (or even absence) co-extraction, and others, make extraction a very effective method for concentrating microimpurities and separating substances. To date, methods have been developed for the extraction of almost all elements and many classes of compounds both for preparative purposes and in technology, especially nuclear technology. Extraction can be used for both absolute and relative concentration. Relative extraction concentration, at which enrichment is achieved, i.e., the ratio between macro- and microcomponents decreases, is more important for analysis. In the practice of chemical analysis, extraction is used either only as a separation method; the isolated element in this case (if necessary, the extract is pre-mineralized) is determined by any conventional method, or in combination with subsequent determination (extraction-photometric, extraction-polarographic and other, so-called combined methods). Determination of the element of interest can be done in both aqueous and organic environments Phase system Driving force of the process Chemical potential gradient Electrical potential gradient Pressure gradient Liquid-liquid-liquid Dialysis through liquid membranesElectrodialysis through liquid membranes - Liquid-solid-liquidDialysis Electrodialysis, electroosmosisUltrafiltration, reverse osmosis, piezodialysis Liquid-solid-gasEvaporation through a membrane - - Gas-solid-gas gas diffusion separation - - Separation methods based on multiple equilibrium distributions always have fundamental limitations on the purity of the isolated fractions. Obviously, good separation in this case can be achieved only if there is a significant difference in the properties of the substances being separated. The process in the field of these methods is entirely determined by the creation of new selective sorbents or extractants. But the emergence of fundamentally new classes of such compounds, such as organophosphorus extractants crown ethers or chiral sorbents, is a relatively rare phenomenon. As a rule, the search leads to the need to obtain increasingly complex and expensive compounds, and the positive effect of their use does not justify the search costs. Based on the data given in the table. 2 classification of methods based on differences in the distribution of substances between phases, one can see another way to increase the efficiency of methods of this group, which consists in improving the methods of carrying out interphase distribution processes, in the transition from single processes to multi-stage and chromatographic ones. It may seem somewhat strange that the chromatographic method is classified as a method for carrying out the process of interfacial distribution of substances. Typically, chromatographic methods are considered in isolation from other separation methods based on differences in the distribution of substances between phases. Numerous definitions of chromatography, proposed over the years, emphasize that chromatography is a separation method or a process leading to the separation of substances. There is no contradiction here. Historically, chromatography arose as a separation method, the essence of which was the separation of zones of individual substances when a solution containing their mixture passes through a layer of solid sorbent. When considering the physicochemical phenomena that cause this “dissolution”, it is natural to speak of chromatography as a process occurring in a two-phase system. When we are talking about a set of methodological techniques applied to almost any two-phase system, which make it possible to greatly increase the separation coefficients achieved with a single equilibrium distribution of substances between phases, we can say that chromatography is a way to carry out the process of interphase separation. Chromatography as a method of carrying out the interphase process consists in the relative movement of phases in a limited space under conditions where one of them is constantly in a dispersed state or in the form of a film on the surface of the walls delimiting this space. This movement occurs in a column, in a capillary, in a thin layer. One of the phases may be stationary or both will be in motion. In any case, the chromatographic method provides multiple sequential redistribution of substances between mutually moving phases, leading to differences in the speeds of movement of the zones of individual substances in the separation space, and in the case of simultaneous movement of both phases in different directions, to differences in the direction of movement of the zones. And the actual separation method will be the application of one of the possible manifestations of the chromatographic method of implementing the process of interphase distribution to a specific two-phase system. The proposed interpretation of the concept of “chromatography” seems essential for understanding the generality of chromatographic separation methods that underlie the most common chromatographic methods of analysis today. Interest in chromatographic methods is determined primarily by the fact that they theoretically have no restrictions on the separation coefficients of substances, no matter how small the differences in distribution coefficients may be for them. This is the qualitative leap that comes from the transition from methods based on a single equilibrium distribution to chromatographic ones. In methods for separating substances based on differences in interfacial distribution, there are always restrictions on mass transfer. More substances cannot move from one phase to another than follows from the distribution coefficient, the value of which, as a rule, decreases with increasing amount of substance in the phase system. The transition to multi-stage and chromatographic methods allows for improved separation, but introduces even more stringent restrictions on the amount of substances to be separated. Thus, for chromatographic methods, the independence of the KD coefficient from concentration becomes a prerequisite, i.e., the requirement for the linearity of the interphase distribution isotherm. Hence, for solving problems that require an increase in mass transfer without increasing the volume of the separating phase, the most promising group of methods is based on induced interphase transfer of matter. We are talking about processes in which separation is carried out under the influence of a constantly acting driving force. In the general case, the implementation scheme of such processes involves the transfer of a substance from one phase to another through a third phase separating them, which is a partition, a membrane. Hence the name of this group of separation methods - membrane methods. In a particular case, there are known attempts to implement induced transfer within a two-phase system - the process of electrical extraction. But since the method is not widely used, and its mechanism is described within one of the stages of the general scheme of the extraction-membrane process, it does not deserve special consideration. The classification by membrane type is a tribute to the history of the development of membrane methods, because their appearance in most cases was initiated by the creation of selectively permeable materials. Only in recent years has there been a trend toward a targeted search for membranes that meet the requirements of a specific membrane separation method. Based on the definition of membrane separation methods as processes of indicated transfer of a substance from one phase to another through a third phase separating them, their main classification features are considered to be the throne system of phases and the driving force of the process (Table 3). Since the main advantage of the membrane scheme for carrying out the separation process is the increase in mass transfer of the substance through the separating phase, membrane methods naturally fall primarily into the sphere of interest of chemical technology. However, a number of interesting areas of application of membrane methods in chemical analysis have already been found, but a mutually enriching exchange of ideas between chemical technology and analytical chemistry in the future cannot be ruled out. Finally, there remains the possibility of separating substances due to differences in the properties of their ions, atoms or molecules, manifested within one homogeneous system under the influence of electric, magnetic, gravitational, thermal fields or centrifugal forces. At the same time, the possibility of phase transformations is not excluded when transferring the initial mixture of substances to the state of aggregation in which separation occurs, or when separating fractions of its individual components. The separation effect is achieved due to different spatial movements of substances within the phase in which their separation occurs. Differences in the speed of spatial movement of ions, atoms or molecules will appear depending on their mass, size, charge, energy of interaction of particles with ions and molecules forming the medium in which separation occurs. The relative role of certain factors in achieving the final separation effect, in turn, depends on the nature of the forces acting on them. The most obvious case is electrophoretic or, as it is sometimes called, electromigration separation of ions in solutions due to the different speeds of their movement in the electric will. The most important factors here are the size and charge of the ion. Differences in mass and charge are most pronounced when ionized particles are exposed to an accelerating electric field and a deflecting magnetic field. This method of influencing the system is the basis of the mass separation method. When separating under the influence of centrifugal forces—ultracentrifugation—the determining factor is the mass of the molecules. Table 4. Intraphase separation methods Aggregate state of the phases in which separation occurs Type of forces causing spatial movement of ions, atoms or molecules Electric field Electric and magnetic field Centrifugal force or gravitational field Liquid Electrophoresis (electromigration) - ultracentrifugation Gas Electrophoresis Mass separation ultracentrifugation Consequently, any of the known methods of intraphase separation can be characterized by the aggregative state of the phase within which the separation occurs and the type of forces causing the spatial movement of ions, atoms or molecules. Intraphase separation methods in general are characterized by complex hardware solutions, and the feasibility of their use in analytical chemistry is justified in proportion to the capabilities that other methods do not have. The simplest technical design is the method of electrophoretic (electromigration) separation of ions in solution, which retains certain areas of application in analytical chemistry. Mass separation as a separation method is interesting primarily because it is the basis of one of the widely used methods of chemical analysis - mass spectrometry. Here there has been an even closer fusion of separation method and final determination methods than in the case of chromatographic methods of analysis. When describing a mass spectrometric method, it is usually not even mentioned that it is one of the combined methods of analysis. The complexity of the equipment and high energy consumption in the mass separation method are compensated by its versatility and practically unlimited separation capacity. General characteristics of concentration methods
The determination of microimpurities is an urgent task due to increased requirements for the purity of materials and the need for analytical monitoring of the environment. To determine trace amounts, only methods that allow the detection of impurities weighing 10 -7-10-8g, and sometimes up to 10 -14d. The most important are physical methods of analysis: atomic adsorption, neutron activation, X-ray fluorescence and some others. The main tasks when determining macrocomponents: 1.The use of very small portions or sample volumes with significant contents of the components being determined; 2.Analysis of samples of large mass or volume to determine the content of trace amounts of substances. To solve the first problem, in addition to the indicated physical methods of analysis, methods of ultramicroanalysis, including ultramicrochemical analysis, are suitable. It is a set of techniques for using special equipment for working with ultra-small volumes of solutions. To solve the second problem, concentration is used as a preliminary operation. It is necessary in cases where it is necessary to increase the concentrations of microcomponents for subsequent analysis or to separate trace amounts of analyte components from the main or other microcomponents. With absolute concentration, microcomponents are transferred from a larger volume to a smaller one. As follows from the definition, concentration is always associated with the separation and redistribution of substances into different phases, therefore all methods suitable for separation are used for concentration. The most common methods are listed in table. 5. when choosing a concentration method, they are guided by the nature of the object and its chemical composition, the subsequent method of analysis, the duration of all operations, the availability of all necessary equipment, etc. Table 5. Concentration methods for trace analysis Method Characteristics and advantages Disadvantages Extraction Allows you to concentrate both impurities of a group of substances and individual substances. The method is universal, simple to design; Use of expensive reagents; Co-precipitation Allows concentration of substances; Long-lasting, less universal, low selectivity; Ion exchange chromatography; Used to exchange the main component of a mixture for H +(OH -) or for concentrating microcomponents from large volumes of solutions. Complete separation is achieved at low values of distribution coefficients. Selectivity is low, losses and contamination are possible due to sorption processes, the process is labor-intensive Distillation It is used to concentrate highly volatile impurities with their condensation on a small surface. Does not require additional reagents and solvents. Application limited to certain classes of substances.
Co-precipitation as a concentration method
Recently, coprecipitation, one of the most effective and long-known methods for concentrating trace amounts of various elements, has become of particular importance for analytical purposes. Co-precipitation is a type of distribution, i.e. the distribution of a microcomponent caused by the release of the reservoir into the solid phase. In other words, it represents the simultaneous transition of micro- to macrocomponents in the forming solid phase of the reservoir. Co-precipitation involves the capture of impurities during the growth of collector particles (introduced into the system as newly formed ones). During Ostwald ripening of sediment, as well as during structural and morphological improvement of solid phase particles. Metal hydroxides (iron, aluminum, etc.), sulfides (CdS, HgS), phosphates (Ca3(PO4)2, etc.), sulfates (BaSO4, etc.) and other inorganic compounds are used as collectors. There are two broad classes of pollution: Co-precipitation, when the main substance and the impurity are deposited together. The fact that two substances precipitate simultaneously does not indicate coprecipitation. Thus, if, for example, traces of beryllium hydroxide are quantitatively precipitated with a large amount of aluminum hydroxide under conditions where both are insoluble, that is, from their saturated solutions, then one should speak of co-precipitation, and not of coprecipitation (conjugate precipitation); Post-deposition is the transition of impurities into sediment not during its formation, but after. First, a pure main precipitate is released, and then an impurity. Typically, post-precipitation occurs in a supersaturated solution. Thus, traces of zinc (II), indium turn into precipitate upon long-term contact of their solutions with the precipitate of metal sulfides. When deposition involving one solid phase, the following cases of coprecipitation are distinguished: Formation of a chemical compound. The gross composition of the solid phase is different from the composition of each of its ingredients, but the local composition is the same. Formation of a solid solution. A change in the gross composition of the solid phase with a change in the concentration of components in the initial mixture indicates the formation of a solid solution. The solid phase resulting from this type of coprecipitation is sometimes called a compound of variable composition. The formation of solid solutions occurs as a result of molecular processes that can be considered as quasi-chemical exchange or addition reactions. The generality of the molecular mechanism of formation is an important argument in favor of combining all cases of coprecipitation with the formation of solid solutions in one subclass. This subclass can be divided into two types depending on whether the solid solution is crystalline or amorphous. Co-precipitation with the formation of a crystalline solid phase is usually called co-crystallization. Formation of a solid phase with an impurity segregated on defects. The gross composition of the solid phase in this case depends on the composition of the initial mixture, and the local composition is not the same. There are two types of segregation: A) During their growth, only the macrocomponents of the initial mixture or the products of their interaction pass into the volume of sediment particles during their growth, and the microcomponent is pushed aside by the growing particles, accumulating near the phase interface (episegregation). This type is associated with the capture of impurities by the surface of growing sediment particles; b) the microcomponent is localized in the volume of the solid phase (endosegregation), near dislocations (dislocation endosegregation), at intercrystalline boundaries (intercrystalline endosegregation) or is located within isolated inclusions of the uterine medium (occlusive endosegregation). In coprecipitation with the participation of several solid phases, a distinction is made between coprecipitation with the participation of separable and inseparable solid phases. If the coprecipitation process occurs either only on the surface of the solid phase, or also inside it, then two types of coprecipitation are distinguished: adsorption - deposition of impurities on the surface of particles; occlusion - deposition of an impurity within the primary particles through any possible mechanism. The phenomenon of coprecipitation is widely used in analytical chemistry as a simple and effective way to extract trace elements from highly dilute solutions in which the solubility product of the precipitate is not achieved. Conclusion Methods of separation and concentration are accustomed to be viewed as something that complements analytical chemistry, expanding its capabilities, but not as its fundamental core part. On the other hand, as already noted in the introduction, one of the two main directions in the development of analytical chemistry is a priori focused on a limited combination of methods for the separation and determination of substances in objects of analysis. Bibliography
1.Moskvin L.N., Tsaritsina L.G. Methods of separation and concentration in analytical chemistry. - L., “Chemistry”, 1991. - 256 p. 2.Skorokhod O.R. Chemical analysis: Fundamentals of methods for concentrating and separating substances. - Mn., "BSU Publishing House", 1980. - 272 p. .Posypaiko V.I. and others. Chemical methods of analysis: Textbook. manual for chemical technologists. universities / Posypaiko V.I., Kozyreva N.A., Logacheva Yu.P. - M., “Higher. school", 1989. - 448 p.Similar works to - Methods of separation and concentration in the analysis of elements
Separation and concentration methods are used to separate complex multicomponent mixtures, isolate the analyte component from the mixture, and increase the concentration of the analyzed component in the sample. These methods include: extraction, separation and concentration by precipitation, evaporation, ashing and zone smelting. These methods are more correctly classified as physicochemical methods, since they are based on such properties of substances as solubility, volatility, melting, crystallization.
The need for separation and concentration when conducting expert research may be due mainly to the following factors:
- the concentration of the component being determined is below the detection limit of the method;
- the test sample contains components that interfere with the determination.
Extraction - the process of extracting individual components of a complex mixture using a solvent. When working with solid samples (finely ground powder), different solubilities of the individual components of the mixture are used, and when extracting from a solution, different distributions of the mixture components in two immiscible liquids are used.
These methods are widely used in the study of forensic objects, both for preliminary research and for preparation for subsequent analysis of such objects as writing materials, paper, paints and varnishes, gunpowder, fibers, identification marks, polymeric materials, drugs and medications, petroleum products and fuels and lubricants, soils and minerals.
Precipitation. Methods of isolation and concentration by precipitation - based on the isolation of components from a mixture in the form of a sparingly soluble compound or co-precipitation on a sparingly soluble precipitate of an inorganic, organic or mixed compound.
Evaporation is a process of separation and purification of substances in which a liquid or solid substance, when heated, becomes a gaseous state (evaporates from the mixture), and then, when cooled, condenses, forming a liquid or sometimes solid phase again.
There are methods: distillation, fractional evaporation (distillation), sublimation.
Distillation or simple evaporation - a one-step process of separating and concentrating substances.
Distillation or fractional evaporation based on different volatility of substances. The separation and concentration of the components of the mixture occurs due to the difference in their boiling points and the evaporation of individual components at different temperatures at different times.
Sublimation (sublimation) – This is the transfer of a substance from a solid to a gaseous state and its subsequent precipitation in solid form (bypassing the liquid phase). For sublimation of microquantities of substances, the “cold finger” method is often used, in which a trace component is condensed on a cooled rod located inside a closed vessel directly above the heated sample; if necessary, the system is evacuated
Ashing methods– these are concentration methods that involve the mineralization of objects of analysis - organic and organometallic compounds, animal and plant materials, soils for subsequent elemental analysis.
They are used for preparing objects for forensic technical examination of documents, forensic biological examination, examination of substances, materials and products made from them, for elemental analysis by chemical and spectral methods. It is possible to differentiate paper of the same type by the color of the ash residue and approximate determination of the type of filler. Mineralization of pharmaceuticals is used for the approximate determination of a medicinal substance by the type of ash residue.
Zone melting is a method for purifying solid heat-resistant substances, based on the redistribution of mixture components in the melt (between the contacting molten liquid and solid phases).
The condition for using the zone melting method is the thermal stability of the substance at the melting temperature and its ability to crystallize. The absence of solvents during the purification process practically eliminates the loss of a substance, possible due to its incomplete separation from the solution.
Electrochemical separation methods are based on the isolation of the analyte on an inert electrode in an elemental state or in the form of an insoluble precipitate that precipitates when a direct electric current is passed through the analyzed solution.
The theory and practice of electrochemical separation methods are described in detail in Chap. VI.
Currently, electrochemical methods are used to separate compounds of most chemical elements and have proven to be very convenient due to the fact that they do not require the introduction of foreign substances into the analyzed solution. Using various methods of electrochemical deposition using platinum or other electrodes and a mercury cathode, as well as internal electrolysis (see Chapter VI, § 5), it is possible to separate cations of aluminum, titanium, zirconium, vanadium, uranium from cations of chromium, iron, cobalt, nickel, zinc, copper, silver, cadmium, germanium, molybdenum, tin, bismuth and other elements. It is also possible to separate impurities from the main components when analyzing non-ferrous metals, their alloys and ores.
The separation of a complex mixture consisting of various electrolytically deposited ions is achieved either by selecting an appropriate electrolyte or by performing electrolysis with automatic control of the electrode potential at which electrodeposition is carried out.
Deposition on a mercury cathode. A special kind! electrolytic separation is deposition on a mercury cathode. In this case, metals under the influence of electric current are released on mercury to form amalgams. As a result, the isolation and separation of many metals occurs quickly and quantitatively. Some metals (for example, ), which are not released on a solid cathode, are quantitatively released during electrolysis on a mercury cathode. However, a number of metals (for example,) are not deposited on the mercury cathode.
Electrolysis with a mercury cathode is carried out in special devices (Fig. 95). To do this, about 200 g of metallic mercury is added to the vessel in which electrolysis is carried out, the solution to be analyzed is poured, and electrolysis is carried out at a current density of about . The anode is strengthened at the surface of the solution. During the electrolysis process, mercury is stirred. At the end of electrolysis, a sample of the solution should give a negative reaction to the separated elements.
Without interrupting the current, the solution is drained from the mercury and the mercury is washed with water. The wash water is added to the main solution. The resulting solution, free of elements isolated at the mercury cathode, is analyzed. If it is necessary to extract metals deposited in it from mercury, the amalgam is dissolved in acids or the mercury is distilled off.
Using a mercury cathode you can separate:
a) aluminum from iron, zinc, copper;
b) titanium from indium, rhenium, molybdenum, germanium;
c) vanadium from molybdenum;
d) phosphorus and arsenic compounds from all elements released at the mercury cathode (iron, zinc, cobalt, nickel, chromium, silver, copper, cadmium, mercury, tin, platinum, gold, etc.).
For example, a method for the quantitative determination of aluminum in the presence of iron ions and other elements, based on the separation of iron by electrolysis on a mercury cathode, is as follows. First, iron is isolated from a sulfate solution at a mercury cathode; along with iron, other elements are released: zinc, chromium, nickel, cobalt, etc. The ions of aluminum, beryllium, titanium, phosphorus, etc. remain in the solution. Then aluminum ions are determined in the usual way, which are precipitated from the filtrate with oxyquinoline or cupferon in ammonia or slightly acetic acid solution. Titanium is precipitated in an acidic solution with cupferon.
Electrochemical methods can be used not only for the separation and isolation of elements, but also for the concentration of analytes.
This kind of concentration (accumulation) of the element being determined on a stationary drop of mercury takes place in the method of amalgam polarography. The essence of the method is that by electrolysis for some time at the potential of the limiting current of the metal being determined, it is concentrated in the form of an amalgam from a dilute solution on a stationary drop of mercury, which acts as a mercury microelectrode. Then, at a linearly varying voltage, the anodic dissolution curve of the amalgam is recorded. In this case, anodic peaks are obtained on the polarogram, the position of which, by potential, characterizes the nature of the substance, and the height of the peak - the concentration of the impurity.
The method was proposed by Barker and studied by Shein, Kemulya, A.G. Stromberg, E.N. Vinogradova, L.N. Vasilyeva, S.I. Sinyakova and others. Great theoretical and practical value for amalgam polarography with preliminary concentration (accumulation) on A stationary drop of mercury is represented by the work of M. T. Kozlovsky in the field of research of metal amalgams.

Rice. 95. Mercury cathode.
The combination of electrochemical concentration of the elements being determined on a stationary drop of mercury with subsequent anodic polarization of the resulting amalgam makes it possible to increase the sensitivity of the method by several orders of magnitude compared to the conventional polarography method. This is of great practical importance for the analysis of highly pure metals, chemical reagents and semiconductor materials.
There are several options for concentrating microimpurities using the electrochemical (polarographic) method. Their general principle is the accumulation of determined elements by preliminary electrolysis on a stationary electrode.
For the purpose of electrochemical preconcentration, amalgam polarography techniques have been developed to determine impurities in zinc, aluminum, indium, tin, arsenic, gallium, uranium salts, chemically pure reagents, biological objects, food products, etc.
Send your good work in the knowledge base is simple. Use the form below
Students, graduate students, young scientists who use the knowledge base in their studies and work will be very grateful to you.
1. Separation and concentration methods
General information about separation and concentration
Separation is an operation that allows the components of a sample to be separated from each other.
It is used if some components of the sample interfere with the determination or detection of others, that is, when the analytical method is not selective enough and overlap of analytical signals must be avoided. In this case, the concentrations of the separated substances are usually close.
Concentration is an operation that allows you to increase the concentration of a microcomponent relative to the main components of the sample (matrix).
It is used if the concentration of a microcomponent is less than the detection limit Cmin, i.e. when the analysis method is not sensitive enough. However, the concentrations of the components vary greatly. Concentration is often combined with separation.
Types of concentration.
1. Absolute: the microcomponent is transferred from a large volume or large mass of the sample (Vpr or mpr) to a smaller volume or smaller mass of the concentrate (Vconc or mconc). As a result, the concentration of the microcomponent increases n times:
where n is the degree of concentration.
The smaller the volume of concentrate, the greater the degree of concentration. For example, 50 mg of cation resin absorbed germanium from 20 L of tap water, then germanium was desorbed by 5 ml of acid. Consequently, the degree of concentration of germanium was:
2. Relative (enrichment): the microcomponent is separated from the macrocomponent so that the ratio of their concentrations increases. For example, in the initial sample the ratio of concentrations of micro- and macrocomponents was 1: 1000, and after enrichment it was 1: 10. This is usually achieved by partial removal of the matrix.
Separation and concentration have much in common; the same methods are used for these purposes. They are very diverse. Next, the methods of separation and concentration that are of greatest importance in analytical chemistry will be considered.
Classification of separation and concentration methods
There are many classifications of separation and concentration methods based on different characteristics. Let's look at the most important of them.
1. Classification according to the nature of the process is given in Fig.
Rice. 1 Classification of separation methods according to the nature of the process
Chemical methods of separation and concentration are based on the occurrence of a chemical reaction, which is accompanied by precipitation of the product and the release of gas. For example, in organic analysis, the main method of concentration is distillation: during thermal decomposition, the matrix is distilled off in the form of CO2, H2O, N2, and metals can be determined in the remaining ash.
Physicochemical methods of separation and concentration are most often based on the selective distribution of a substance between two phases. For example, in the petrochemical industry, chromatography is of greatest importance.
Physical methods of separation and concentration are most often based on changing the state of aggregation of a substance.
2. Classification according to the physical nature of the two phases. The distribution of a substance can be carried out between phases that are in the same or different states of aggregation: gaseous (G), liquid (L), solid (S). In accordance with this, the following methods are distinguished (Fig.).
Rice. 2 Classification of separation methods according to the nature of the phases
In analytical chemistry, methods of separation and concentration, which are based on the distribution of a substance between the liquid and solid phases, have found the greatest importance.
3. Classification according to the number of elementary acts (stages).
§ Single-stage methods - based on a single distribution of a substance between two phases. The separation takes place under static conditions.
§ Multistage methods - based on multiple distribution of a substance between two phases. There are two groups of multi-stage methods:
– repeating the single distribution process (for example, repeated extraction). The separation takes place under static conditions;
– methods based on the movement of one phase relative to another (for example, chromatography). Separation takes place under dynamic conditions
3. Classification according to the type of equilibrium (Fig.).
Rice. 3 Classification of separation methods by type of equilibrium
Thermodynamic separation methods are based on differences in the behavior of substances in an equilibrium state. They are of greatest importance in analytical chemistry.
Kinetic separation methods are based on differences in the behavior of substances during the process leading to an equilibrium state. For example, in biochemical research, electrophoresis is of greatest importance. Other kinetic methods are used to separate particles of colloidal solutions and solutions of high molecular weight compounds. In analytical chemistry, these methods are used less frequently.
Chromatographic methods are based on both thermodynamic and kinetic equilibrium. They are of great importance in analytical chemistry, since they allow the separation and simultaneous qualitative and quantitative analysis of multicomponent mixtures.
Extraction as a method of separation and concentration
Extraction is a method of separation and concentration based on the distribution of a substance between two immiscible liquid phases (most often aqueous and organic).
For the purpose of extraction separation, conditions are created such that one component completely passes into the organic phase, and the other remains in the aqueous phase. The phases are then separated using a separating funnel.
For the purpose of absolute concentration, the substance is transferred from a larger volume of aqueous solution to a smaller volume of the organic phase, as a result of which the concentration of the substance in the organic extract increases.
For the purpose of relative concentration, conditions are created so that the microcomponent passes into the organic phase, and the majority of the macrocomponent remains in the aqueous phase. As a result, in the organic extract the ratio of the concentrations of the micro- and macrocomponents increases in favor of the microcomponent.
Advantages of extraction:
§ high selectivity;
§ ease of implementation (only a separating funnel is needed);
§ low labor intensity;
§ speed (3-5 min);
§ extraction combines very well with methods of subsequent determination, as a result of which a number of important hybrid methods have emerged (extraction-photometric, extraction-spectral, etc.).
Co-precipitation as a method of separation and concentration
Co-precipitation is the capture of a microcomponent by a sediment-collector during its formation, and the microcomponent passes into the sediment from an unsaturated solution (PS< ПР).
Inorganic and organic poorly soluble compounds with a developed surface are used as collectors. Phase separation is carried out by filtration.
Co-precipitation is used for the following purposes:
§ concentration of impurities as a very effective and one of the most important methods, which allows you to increase the concentration by 10-20 thousand times;
§ separation of impurities (less often).
Sorption as a method of separation and concentration
Sorption is the absorption of gases or dissolved substances by solid or liquid sorbents.
Activated carbons, Al2O3, silica, zeolites, cellulose, natural and synthetic sorbents with ionogenic and chelating groups are used as sorbents.
The absorption of substances can occur on the surface of the phase (adsorption) or in the volume of the phase (absorption). In analytical chemistry, adsorption is most often used for the purpose of:
§ separation of substances, if conditions for selective absorption are created;
§ concentration (less often).
In addition, sorption under dynamic conditions forms the basis for the most important method of separation and analysis - chromatography.
Ion exchange
Ion exchange is a reversible stoichiometric process that occurs at the interface between the ionite and the electrolyte solution.
Ion exchangers are high-molecular polyelectrolytes of various structures and compositions. concentration chemical sorption gas
The main property of ion exchangers is that they absorb cations or anions from a solution, releasing into the solution an equivalent number of ions of the same charge sign.
The process of ion exchange is described by the law of mass action:
where A and B are ions in solution, and are ions in the ion exchanger phase.
This equilibrium is characterized by the exchange constant (K):
where a is the activity of ions.
If K > 1, then the B ion has a greater affinity for the ion exchanger; if K< 1, то ион А обладает бульшим сродством к иониту; если же К? 1, то оба иона одинаково сорбируются ионитом.
The following factors influence the course of ion exchange:
1) the nature of the ion exchanger;
2) the nature of the ion: the greater the ratio of the ion charge to the radius of the hydrated ion (z/r), the greater the affinity for the ion exchanger;
3) properties of the solution:
§ pH value (see the following sections);
§ ion concentration: from dilute solutions, the ion exchanger sorbs ions with a larger charge, and from concentrated solutions - with a smaller one;
§ ionic strength of the solution: the smaller the m, the better the ions are sorbed.
Types of ion exchangers
There is a large number of different ion exchangers. They are classified according to their origin and the sign of the charge of the exchanging ions.
Depending on the origin, two groups are distinguished
ion exchangers:
1. Natural ion exchangers:
§ inorganic (clays, zeolites, apatites);
§ organic (cellulose).
2. Synthetic ion exchangers:
§ inorganic (permutites);
§ organic (high molecular weight materials).
In analytical chemistry, synthetic organic ion exchangers are most often used.
Depending on the sign of the charge of the exchanging ions, ion exchangers are called as follows:
1. Cation exchangers - exchange cations, contain acid groups:
§ -SO3H (strong acid cation exchangers, exchange occurs in a wide range of pH values);
§ -PO3H2 (medium acid cation exchangers, exchange occurs at pH > 4);
§ -COOH, -OH (weak acid cation exchangers, exchange occurs at pH > 5).
2. Anion exchangers - exchange anions, contain basic groups:
§ quaternary alkylammonium groups (highly basic anion exchangers, exchange occurs in a wide range of pH values);
§ amino and imino groups (medium and low basic anion exchangers, exchange occurs at pH< 8-9).
3. Ampholytes - exchange both cations and anions depending on conditions. They have both types of groups - acidic and basic.
Structure of synthetic organic ion exchangers. Ion exchange reactions
Synthetic organic ion exchangers have a three-dimensional chain structure. They consist of a high molecular weight (HM) matrix in which ionogenic groups are fixed.
For example, for a highly basic anion exchanger in the chloride form R-N(CH3)3Cl
Composition of the ion exchanger
|
stationary HM ion |
mobile NM ion |
||
|
fixed ion |
counterion |
||
|
ionic group |
The matrix is usually a copolymer of styrene and divinylbenzene (DVB), which is a cross-linking agent: each of its molecules, like a bridge, connects 2 adjacent linear polystyrene chains.
Mobile low molecular weight (LM) ions that are part of ionogenic groups participate in ion exchange.
For example, a cation exchange reaction involving a strongly acidic cation exchanger in hydrogen form is written as follows:
and an anion exchange reaction involving a highly basic anion exchanger in chloride form
Basic physical and chemical characteristics of ion exchangers
Ionites as materials have many physical-chemical and physical-mechanical characteristics. Of these, three main physical and chemical characteristics are of greatest importance to the analytical chemist - moisture, swelling and exchange capacity.
Humidity (W, %) characterizes the ability of the ion exchanger to absorb moisture from the air. It can be calculated based on experimental data:
where mo and m are the mass of the ion exchanger before and after drying.
Typically, the humidity of ion exchangers is in the range of 10-15%.
Swelling characterizes the degree of increase in the volume of the ion exchanger upon contact with water or other solvent. The amount of swelling depends on the degree of cross-linking of the high molecular weight ion exchanger matrix (% DVB). Due to swelling, ion exchange occurs quickly. The reason for swelling is the presence of polar ionogenic groups capable of hydration or solvation. Exchange capacity (EC) is the most important quantitative characteristic of an ion exchanger. It characterizes the ability of the ion exchanger to ion exchange. The total exchange capacity (TEC) of a given ion exchanger is a constant value and is determined by the number of fixed ions in the ion exchanger matrix. It depends on the following factors: the nature of the ion exchanger;
§ solution pH value;
§ definition conditions (static or dynamic);
§ nature of the exchanged ion;
§ ion radius (sieve effect).
Mass exchange capacity shows how many millimole equivalents of an ion - n(1/z ion) - can exchange 1 gram of dry ion exchanger. It is calculated using the formula:
Volumetric exchange capacity shows how many millimole equivalents of an ion - n(1/z ion) - can exchange 1 milliliter of swollen ion exchanger. It is calculated using the formula:
Depending on the determination conditions, a distinction is made between static (SOE) and dynamic (DOE) exchange capacity, and what about SOE? DOE.
Types of dynamic exchange capacity:
§ before the breakthrough of the absorbed ion, or working (DOE), shows how many ions can be absorbed by the ion exchanger before they appear in the eluate (breakthrough);
§ total (PDOE) - shows how many ions can be absorbed by the ion exchanger until the ionogenic groups are completely saturated under given conditions.
The difference between the values of DOE and PDOE is presented in the figure:
Posted on http://www.allbest.ru/
Rice. 4 Total dynamic exchange capacity (TDEC) and capacity before breakthrough (DEC)
Application of ion exchangers in analytical chemistry
Ion exchangers are used to solve the following problems in analytical practice.
§ Separation of substances. Ion exchange is a convenient and effective method for separating substances. For example, with its help it is possible to separate even elements with similar chemical properties, such as lanthanides.
§ Concentration of substances. First, a large volume of the dilute solution is passed through a column containing an ion exchange resin. After this, the sorbed ions are washed out of the column with a minimum amount of a suitable eluent.
§ Determination of “inconvenient” cations and anions. It is often necessary to analyze the content of so-called “inconvenient” ions. Such ions do not have chemical analytical properties that would allow them to be easily determined using chemical or instrumental methods of analysis. Of the cations, these include ions of alkali metals (Na+, K+, etc.), of anions - , etc. The determination of “inconvenient” cations is based on first passing the sample through a column with a cation exchanger in hydrogen form and subsequent titration with alkali:
The determination of “inconvenient” anions is based on preliminary passing the sample through a column with an anion exchanger in hydroxide form and subsequent titration of the released alkali with an acid:
§ Obtaining deionized water. Water is passed sequentially through a column with a cation exchange resin in the hydrogen form, then through a column with an anion exchange resin in the hydroxide form. As a result, all cations and anions are retained by ion exchangers and water is obtained that does not contain ions.
Chromatographic methods of analysis
The chromatographic method of analysis was first used by the Russian botanist M. S. Tsvet for the analysis of chlorophyll. The name of the method comes from the Greek word “chromatos” - color, although the method allows you to separate any compounds, including uncolored ones.
Currently, chromatography is one of the most promising methods of analysis. It is widely used in various industries and scientific research for the analysis of mixtures of gaseous, liquid and solid substances.
In the petrochemical and gas industry, chromatography accounts for 90% of all analyzes performed. Gas chromatography is used in biology and medicine, wood processing technology, forest chemistry and food industry and other areas. About 30% of environmental monitoring analyzes (air pollution, wastewater analysis, etc.) are performed using gas chromatographic methods.
The essence of chromatographic methods of analysis
Chromatography is a dynamic method for the separation and determination of substances, based on the multiple distribution of components between two phases - mobile and stationary.
The substance enters the sorbent layer along with the flow of the mobile phase. In this case, the substance is sorbed and then, upon contact with fresh portions of the mobile phase, desorbed. The movement of the mobile phase occurs continuously, so sorption and desorption of the substance occur continuously. In this case, part of the substance is in the stationary phase in a sorbed state, and part is in the mobile phase and moves with it. As a result, the speed of movement of the substance is less than the speed of movement of the mobile phase. The more a substance is sorbed, the slower it moves.
If a mixture of substances is chromatographed, then the speed of movement of each of them is different due to different affinities for the sorbent, as a result of which the substances are separated: some components are delayed at the beginning of the journey, others move further.
Classification of chromatographic methods of analysis
Chromatographic methods of analysis are so diverse that there is no single classification of them. Most often, several classifications are used, which are based on the following characteristics:
§ state of aggregation of the mobile and stationary phases;
§ mechanism of interaction of the substance with the sorbent;
§ analysis technique (way of designing the process);
§ chromatography method (method of moving a substance through a column);
§ purpose of chromatography.
Depending on the state of aggregation of the phases, a distinction is made between gas chromatography (mobile phase - gas or vapor) and liquid chromatography (mobile phase - liquid).
According to the mechanism of interaction of a substance with a sorbent, the following types of chromatography are distinguished: adsorption, distribution, ion exchange, sedimentation, redox, complexing, etc.
IN dependencies from way registration process differentiate columnar And planar chromatography. IN columnar chromatography process separation lead V columns, filled sorbent. Planar chromatography includes V myself two varieties: chromatography on paper And thin-layer chromatography on records.
IN dependencies from way chromatography differentiate following kinds chromatography:
§ eluent (developing) chromatography;
§ repressive chromatography;
§ frontal chromatography.
More often In general, the development method of chromatography is used. It consists in introducing into a continuous flow of the mobile phase (eluent) a mixture of substances that are sorbed better than the eluent. As the eluent moves through the column with sorbed substances, they move along the sorbent layer at different speeds and finally leave it in separate zones separated by the eluent.
According to the purpose of the chromatographic process, they are distinguished: analytical chromatography - an independent method of separation, qualitative and quantitative analysis of substances; preparative chromatography to isolate pure substances from a mixture.
Gas chromatography
The gas chromatography method has become most widespread because the theory and equipment for it have been most fully developed.
Gas chromatography is a hybrid method that allows simultaneous separation and determination of the components of a mixture.
Gases, their mixtures or compounds that are in the gaseous or vapor state under separation conditions are used as the mobile phase (carrier gas).
Solid sorbents (gas adsorption chromatography) or liquid applied in a thin layer to the surface of an inert carrier (gas-liquid chromatography) are used as a stationary phase.
Advantages of analytical gas chromatography:
§ the ability to identify and quantify individual components of complex mixtures;
§ high clarity of separation and expressiveness;
§ the ability to study microsamples and automatically record results;
§ the ability to analyze a wide range of objects - from light gases to high-molecular organic compounds;
Main theoretical approaches
The task of the theory of chromatography is to establish the laws of motion and blurring of chromatographic zones. Most often, the following approaches are used for this:
§ theory of theoretical plates;
§ kinetic theory.
The theory of theoretical plates is based on the assumption that the column is divided into small sections - plates. These are narrow layers of the column in which equilibrium is established in the distribution of the substance between the mobile and stationary phases.
The kinetic theory relates the efficiency of separation to the processes of diffusion of the substance in the column due to the movement of the carrier gas flow. When a substance moves along the column, it is either in the mobile phase or in the stationary phase, i.e., the chromatography process is stepwise. The time a substance spends in both phases determines the speed of its movement through the column.
Chromatographic Peak Parameters
Rice. 5 Chromatogram of a mixture of three substances
1. Retention time (tR) is the time from the moment the analyzed sample is introduced until the maximum of the chromatographic peak is recorded. It depends on the nature of the substance and is a qualitative characteristic.
2. Height (h) or area (S) of the peak
S = ½ h. (4)
The height and area of the peak depend on the amount of substance and are quantitative characteristics.
The retention time consists of two components - the residence time of substances in the mobile phase (tm) and the residence time in the stationary phase (ts):
Schematic diagram of a gas chromatograph and the purpose of the main components
The sample injection device 3 allows a certain amount of the analyzed mixture in a gaseous state to be introduced into the carrier gas flow immediately before the column. It includes an evaporator and a dosing device.
The carrier gas flow introduces the analyzed sample into column 5, where the mixture is separated into its individual components.
Rice. 6 Block diagram of a gas chromatograph: 1 - cylinder with carrier gas; 2 - gas preparation unit; 3 - sample injection device; 4 - thermostat; 5 - chromatographic column; 6 - detector; 7 - amplifier; 8 - recorder
The latter, in a mixture with a carrier gas, are supplied to the detector 6, which converts the corresponding changes in the physical or physico-chemical properties of the mixture of components - carrier gas compared to pure carrier gas into an electrical signal. A detector with a corresponding power supply makes up the detection system.
The required temperature conditions of the evaporator, column and detector are achieved by placing them in the corresponding thermostats 4, controlled by a thermostat. If it is necessary to increase the column temperature during analysis, use a temperature programmer. Thermostats and a thermostat with a programmer make up a thermostating system, which also includes a device for measuring temperature.
The detector signal, converted by amplifier 7, is recorded in the form of a chromatogram by recorder 8.
Often an electronic integrator or data processing computer is included in the circuit.
Conditions for chromatographic analysis
When carrying out chromatographic analysis, it is necessary to select optimal conditions for the separation of the analyzed components. As a rule, when determining them, they are guided by literature data. Based on them, the following are experimentally selected:
§ stationary phase in gas-liquid or adsorbent in gas adsorption chromatography;
§ solid inert carrier in gas-liquid chromatography;
§ carrier gas;
§ carrier gas consumption;
§ sample volume;
§ column temperature.
Qualitative analysis
Basic methods of identifying substances:
1. Tag method
The first version of the method is based on the fact that, under the same conditions, the retention times of the reference (label) and analyte substances are experimentally determined and compared. Equality of retention parameters allows the substance to be identified.
The second version of the labeling method is that a reference component (label), the presence of which is assumed to be in the mixture, is introduced into the mixture being analyzed. An increase in the height of the corresponding peak compared to the height of the peak before the addition of the additive indicates the presence of this compound in the mixture.
2. Use of literature values of retention parameters.
Quantitative Analysis
Quantitative analysis is based on the dependence of the peak area on the amount of substance (in some cases, the peak height is used).
There are various ways to determine peak area:
§ according to the formula, as the area of a triangle;
§ using a planimeter;
§ weighing the cut out peaks (the peaks in the chromatogram are copied onto uniform paper, cut out and weighed);
§ using an electronic integrator;
§ using a computer.
The accuracy of quantitative chromatographic analysis is largely determined by the choice of the most rational method for calculating the concentration of substances. The main methods are:
§ absolute calibration method,
§ internal normalization method,
§ internal standard method.
Absolute calibration method
The essence of the method is that known quantities of a standard substance are introduced into a chromatographic column and the peak areas are determined.
Based on the data obtained, a calibration graph is constructed. Then the analyzed mixture is chromatographed and the content of this component is determined according to the graph.
To calculate these coefficients, the peak areas of at least 10 standard mixtures with different contents of a given substance i are determined. Then use the formula.
ki = shi q / (S 100),
where ki is the absolute correction factor of the i-th substance; ui is the content of the i-th component in the standard mixture (%); S - peak area;
q is the sample size (volume, cm3 - for gases, μL - for liquids, or mass, μg - for liquids and solids).
The coefficients obtained in this way are averaged. Then the test mixture is analyzed and the result is calculated using the formula
shi = ki S 100/q.
The absolute calibration method is quite simple, but the necessary conditions for its use are the accuracy and reproducibility of sample dosing, strict adherence to the constancy of the chromatographic mode parameters when calibrating the device and when determining the content of the chromatographed substance.
The absolute calibration method is especially widely used when determining one or more components of a mixture, in particular when using a chromatograph to regulate the technological process mode based on the content of one or a small number of substances in products. This method is the main one for determining trace impurities.
Relative correction factors
Due to the low accuracy of sample dosing, a number of methods have been developed in which the sample size is not used in calculations. These methods use relative correction factors. They take into account differences in the sensitivity of the detector used to the components of the analyzed sample and depend little on process parameters. They are found in advance for each component of the sample.
To determine relative correction (calibration) coefficients, a series of binary mixtures of known composition are prepared and, based on the resulting chromatograms, calculations are carried out using the formula
ki =(i /st)/(Si/Sst), (4)
You can use calibration mixtures from a larger number of substances, however, the accuracy of the determination may decrease.
Relative correction factors are used in the methods of internal normalization, internal standard, etc.
Internal normalization method
The essence of the method is that the sum of the peak areas of all components of the mixture is taken as 100%.
A necessary condition for using the method is the registration of all components (the chromatogram contains separated peaks of all components of the mixture).
The concentration of the i-th component is calculated using the formula
i = ki Si 100/ У(ki Si).
When calculating correction factors using formula (4) for this method, one of the compounds included in the mixture under study can be selected as a standard. The calibration factor for a standard substance is equal to 1.
Internal standard method
The essence of the method is that a certain amount of a standard substance (comparison substance) is introduced into the analyzed mixture.
i = ki Si 100 r/Sst..
where ki is the relative correction factor of the i-th component, calculated according to formula (4); Si and Sst. - peak areas of the i-th component and internal standard; r is the ratio of the mass of the internal standard to the mass of the analyzed mixture (without standard): r = mst./mmixture.
Requirements for a substance used as an internal standard:
§ it should not be part of the mixture being tested;
§ it must be inert with respect to the components of the mixture being analyzed and completely miscible with them;
§ The peak of the standard must be well resolved and located in close proximity to the peaks of the compounds being determined.
The internal standard is selected from among compounds that are similar in structure and physicochemical properties to the components of the mixture being analyzed. Relative correction factors for mixture components are determined in relation to the internal standard.
The method is used both when all components of the analyzed mixture are recorded on the chromatogram, and in the case of incompletely identified mixtures. The main difficulty lies in the selection and precise dosage of the standard substance.
Posted on Allbest.ru
...Similar documents
Analysis of methods for separating substances as a set of chemical and physical processes characteristic of them and methods of their implementation: extraction, membrane, intraphase. Coprecipitation is a method of concentrating trace amounts of various elements.
course work, added 10/16/2011
General approaches to the synthesis of technological separation schemes. Multivariate organization of the technological separation process. Methods for the synthesis of technological separation schemes. Integral-hypothetical method. Products of separation. Chlorobenzene and dichlorobenzenes.
thesis, added 01/04/2009
Methods for qualitative analysis of substances. Magnetic separation of iron and sulfur and synthesis of iron sulfide. Flotation, filtration and evaporation of mixtures. The use of chromatography as a method of separation and purification of substances. Physical and chemical methods of analysis.
abstract, added 02/15/2016
General approaches to the synthesis of technological separation schemes. Multivariate organization of the technological separation process. Optimization criteria. Methods for the synthesis of technological separation schemes. Synthesis methods based on heuristic rules.
thesis, added 01/04/2009
Physico-chemical characteristics of cobalt. Complex zinc compounds. Study of sorption concentration of Co in the presence of zinc from chloride solutions in an ion exchanger outfit. The technical result that was achieved in the implementation of the invention.
abstract, added 10/14/2014
Sorbents immobilized by substances are a new class of effective sorbents. 8-hydroxyquinoline and its analytical applications. Chelating sorbents with 8-hydroxyquinoline groups. Study of Cu concentration on anion exchanger AV-17 and its results.
course work, added 09/27/2010
The chromatographic method for separating and analyzing complex mixtures was discovered by the Russian botanist M.S. Color. Chromatography is the repeated repetition of acts of sorption and desorption of a substance as it moves in a flow of the mobile phase along a stationary sorbent.
course work, added 03/13/2011
Methods for separating azeotropic and zeotropic mixtures. Azeotropic and heteroazeotropic rectification. Extractive rectification. Methods for the synthesis of technological separation schemes. Some properties, toxic effects, preparation and use of components.
thesis, added 01/04/2009
Equation of a chemical reaction using the electron-ion method. Determination of the potentials of the oxidizer and reducer, the direction of the process, the thermodynamic characteristics of H, S, G. Electronic formula of elements by 2 and 4 quantum numbers.
course work, added 11/25/2009
The thermal effect of a chemical reaction or the change in enthalpy of a system due to the occurrence of a chemical reaction. Influence of external conditions on chemical equilibrium. The influence of pressure, concentration and temperature on the equilibrium position. Types of chemical bonds.