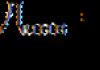m-eng.ru
The textbook is intended for students of non-chemical specialties of higher educational institutions. It can serve as a guide for individuals independently studying the basics of chemistry, and for students of chemical technical schools and senior high schools.
A legendary textbook, translated into many languages of Europe, Asia, Africa and published in a total circulation of over 5 million copies.
When producing the file, the site http://alnam.ru/book_chem.php was used
| <<< Назад
|
Book: |
Forward >>> To determine the state of an electron in a multi-electron atom, the position formulated by W. Pauli is important ( Pauli principle ), Whereby an atom cannot have two electrons whose four quantum numbers are the same . It follows from this that each atomic orbital, characterized by certain values of n, l and m, can be occupied by no more than two electrons whose spins have opposite signs. Two such electrons located in the same orbital and having opposite spins are called paired , as opposed to single (i.e. unpaired
) electron occupying an orbital.
Using the Pauli principle, we calculate the maximum number of electrons that can be at different energy levels and sublevels in an atom.
When l=0, i.e. at the s-sublevel, the magnetic quantum number is also zero. Consequently, at the s-sublevel there is only one orbital, which is conventionally designated as a cage (“quantum cell”): ?.
As mentioned above, each atomic orbital contains no more than two electrons, the spins of which are in opposite directions. This can be symbolically represented by the following diagram:
So, the maximum number of electrons at the s-sublevel of each electronic layer is 2. At l=1 (p-sublevel), three different values of the magnetic quantum number are possible (-1, 0, +1). Hence. The p sublevel has three orbitals, each of which can be occupied by no more than two electrons. In total, the p-sublevel can accommodate 6 electrons:
![]()
Finally, the f sublevel (l=3) can accommodate 14 electrons; in general, the maximum number of electrons at a sublevel with orbital quantum number l is 2(2l+1).
The first energy level (K-layer, n=1) contains only the s-sublevel, the second energy level (L-layer, n=2) consists of s- and p-sublevels, etc. Taking this into account, we will compile a table of the maximum number of electrons located in various electronic layers (Table 2).
As shown in the table. 2 data, the maximum number of electrons at each energy level is 2n 2, where n is the corresponding value of the principal quantum number. So, in the K-layer there can be a maximum of 2 electrons (2 1 2 = 2), in the L-layer - 8 electrons (2 2 2 = 8), in the M-layer - 18 electrons (2 3 2 = 18 ) etc. Note that the resulting numbers coincide with the numbers of elements in the periods of the periodic system.
The most stable state of an electron in an atom corresponds to the minimum possible value of its energy. Any other state of it is excited, unstable: from it the electron spontaneously goes into a state with a lower energy. Therefore, in an unexcited hydrogen atom (nuclear charge Z = 1), the only electron is in the lowest possible energy state, i.e. at the 1s sublevel. The electronic structure of the hydrogen atom can be represented by the diagram
or write it like this: 1s 1 (read “one es one”).
Table 2. Maximum number of electrons at atomic energy levels and sublevels

In a helium atom (Z = 2) the second electron is also in the 1s state. Its electronic structure (1s 2 - read “one es two”) is depicted by the diagram:
This element completes the filling of the K-layer closest to the nucleus and thereby completes the construction of the first period of the electron system.
For the next element after helium, lithium (Z=3), the third electron can no longer fit in the K-layer orbital: this would contradict the Pauli principle. Therefore, it occupies the s-state of the second energy level (L-layer, n=2). Its electronic structure is written by the formula 1s 2 2s 1, which corresponds to the scheme:

The number and relative arrangement of quantum cells in the last diagram shows that 1) electrons in a lithium atom are located at two energy levels, the first of which consists of one sublevel (1s) and is completely filled; 2) the second - external - energy level corresponds to a higher energy and consists of two sublevels (2s and 2p); 3) the 2s sublevel includes one orbital in which there is one electron in the lithium atom; 4) the 2p sublevel includes three energetically equivalent orbitals, which correspond to a higher energy than the energy corresponding to the 2s orbital; in an unexcited lithium atom, the 2p orbitals remain unoccupied.
In the future, for simplicity, in electronic circuits we will indicate only energy levels that are not fully occupied. In accordance with this, the structure of the electron shell of the atom of the next element of the second period - beryllium (Z = 4) - is expressed by the diagram

or the formula 1s 2 2s 2. Thus, as in the first period, the construction of the second period begins with elements in which s-electrons of a new electron layer first appear. Due to the similarity in the structure of the outer electronic layer, such elements also exhibit many similarities in their chemical properties. Therefore, they are usually classified as belonging to the general family s-elements.
The electronic structure of the atom of the element next to beryllium - boron (Z = 5) is depicted by the diagram

and can be expressed by the formula 1s 2 2s 2 2p 1.
When the nuclear charge increases by another unit, i.e. when moving to carbon (Z=6), the number of electrons at the 2p sublevel increases to 2: the electronic structure of the carbon atom is expressed by the formula 1s 2 2s 2 2p 2. However, any of three schemes could correspond to this formula:

According to scheme (1), both 2p electrons in a carbon atom occupy the same orbital, i.e. their magnetic quantum numbers are the same, and their spin directions are opposite; scheme (2) means that 2p electrons occupy different orbitals (i.e., have different m values) and have opposite spins; finally, from diagram (3) it follows that the two 2p electrons correspond to different orbitals, and the spins of these electrons are directed in the same way.
Analysis of the atomic spectrum of carbon shows that for an unexcited carbon atom, it is the last scheme that corresponds to the highest possible value of the total spin of the atom (this is the name of the sum of the spins of all electrons included in the atom; for carbon atom schemes (1) and (2) this sum is zero , and for scheme (3) is equal to one).
This arrangement of electrons in a carbon atom is a special case of the general pattern expressed Hund's rule: The stable state of the atom corresponds to such a distribution of electrons within the energy sublevel at which the absolute value of the total spin of the atom is maximum.
Note that Hund's rule does not prohibit a different distribution of electrons within a sublevel. It only states that sustainable, i.e. unexcited a state in which the atom has the lowest possible energy; with any other distribution of electrons, the energy of the atom will be greater, so that it will be in excited, unstable condition.
Using Hund's rule, it is not difficult to draw up a diagram of the electronic structure for the atom of the element next to carbon - nitrogen (Z = 7):

This scheme corresponds to the formula 1s 2 2s 2 2p 3.
Now that each of the 2p orbitals is occupied by one electron, the pairwise placement of electrons in the 2p orbitals begins. The oxygen atom (Z=8) corresponds to the electronic structure formula 1s 2 2s 2 2p 4 and the following diagram:

The fluorine atom (Z=9) gains one more 2p electron. Its electronic structure is expressed, therefore, by the formula 1s 2 2s 2 2p 5 and the diagram:

Finally, the neon atom (Z=10) finishes filling the 2p sublevel, and thereby completing the second energy level (L-layer) and building the second period of the system of elements.
Thus, starting with boron (Z=5) and ending with neon (Z=10), the p-sublevel of the outer electronic layer is filled;; the elements of this part of the second period therefore belong to the family of p-elements.
The sodium (Z=11) and magnesium (Z=12) atoms, like the first elements of the second period - lithium and beryllium - contain one or two s-electrons in the outer layer, respectively. Their structure corresponds to the electronic formulas 1s 2 2s 2 2p 6 3s 1 (sodium) and 1s 2 2s 2 2p 6 3s 2 (magnesium) and the following schemes:



and the formula 1s 2 2s 2 2p 6 3s 2 3p 6.
Thus the third period, like the second, begins with two s-elements, followed by six p-elements. The structure of the outer electronic layer of the corresponding elements of the second and third periods is therefore similar. Thus, lithium and sodium atoms have one s-electron in their outer electron layer, nitrogen and phosphorus atoms have two s-electrons and three p-electrons, etc. In other words, as the charge of the nucleus increases, the electronic structure of the outer electronic layers of atoms periodically repeats itself. Below we will see that this is also true for elements of subsequent periods. It follows that the arrangement of elements in the periodic table corresponds to the electronic structure of their atoms. But the electronic structure of atoms is determined by the charge of their nuclei and, in turn, determines the properties of the elements and their compounds. This is the essence of the periodic dependence of the properties of elements on the charge of the nucleus of their atoms, expressed by the periodic law.
Let us continue our consideration of the electronic structure of atoms. We settled on the argon atom, whose 3s and 3p sublevels are completely filled, but all the orbitals of the 3d sublevel remain unoccupied. However, in the elements following argon - potassium (Z = 19) and calcium (Z = 20) - the filling of the third electronic layer temporarily stops and the s-sublevel of the fourth layer begins to form: the electronic structure of the potassium atom is expressed by the formula 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1, calcium atom - 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 and the following schemes:


The reason for this sequence of filling electronic energy sublevels is as follows. As indicated in § 31, the energy of an electron in a multi-electron atom is determined by the values of not only the principal, but also the orbital quantum number. The sequence of arrangement of energy sublevels corresponding to an increase in electron energy was also indicated there. The same sequence is shown in Fig. 22.
As Fig. 22, the 4s sublevel is characterized by a lower energy than the 3d sublevel, which is associated with stronger screening of d electrons compared to s electrons. In accordance with this, the placement of outer electrons in potassium and calcium atoms on the 4s sublevel corresponds to the most stable state of these atoms.
The sequence of filling atomic electron orbitals depending on the value of the principal and orbital quantum numbers was studied by the Soviet scientist V.M. Klechkovsky, who established that the electron energy increases as the sum of these two quantum numbers increases, i.e. quantities (n+l). In accordance with this, he formulated the following position (Klechkovsky’s first rule): As the charge of the atomic nucleus increases, the sequential filling of electron orbitals occurs from orbitals with a smaller value of the sum of the principal and orbital quantum numbers (n+l) to orbitals with a larger value of this sum.
The electronic structure of potassium and calcium atoms corresponds to this rule. Indeed, for 3d orbitals (n=3, l=2) the sum (n+l) is equal to 5, and for a 4s orbital (n=4, l=0) it is equal to 4. Therefore, the 4s sublevel should be filled earlier than the 3d sublayer, which is what actually happens.
So, the construction of the 4s sublevel is completed at the calcium atom. However, when moving to the next element - scandium (Z=21) - the question arises: which of the sublevels with the same amount (n+l) - 3d (n=3, l=2), 4p (n=4, l=1) or 5s (n=5, l=0) - must be filled in? It turns out that for the same sum values (n+l), the higher the value of the principal quantum number n, the higher the electron energy. Therefore, in such cases, the order of filling energy sublevels with electrons is determined Klechkovsky's second rule, Whereby at the same values of the sum (n+l), the filling of the orbitals occurs sequentially in the direction of increasing the value of the principal quantum number n.

Rice. 22. Sequence of filling electronic energy sublevels in an atom.
According to this rule, in the case of (n+l) = 5, sublevel 3d (n=3) must be filled first, then sublevel 4p (n=4) and, finally, sublevel 5s (n=5). The scandium atom, therefore, should begin to fill its 3d orbitals, so that its electronic structure corresponds to the formula 1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 4s 2 * and the scheme:

The filling of the 3d sublevel continues in the elements following scandium - titanium, vanadium, etc. - and completely ends in zinc (Z = 30), the atomic structure of which is expressed by the diagram

which corresponds to the formula 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2.
* In electronic structure formulas, it is customary to first write sequentially all states with a given value of n, and then move on to states with a higher value of n. Therefore, the order of recording does not always coincide with the order of filling the energy sublevels. Thus, in the electronic formula of the scandium atom, the 3d sublevel is placed before the 4s sublevel, although these sublevels are filled in the reverse order.
Ten d-elements, starting with scandium and ending with zinc, belong to the transition elements. The peculiarity of the construction of the electron shells of these elements in comparison with the previous ones (s- and p-elements) is that when moving to each subsequent d-element, a new electron appears not in the outer one (n=4), but in the second one outside (n= 3) electronic layer. In this regard, it is important to note that the chemical properties of elements are primarily determined by the structure of the outer electronic layer of their atoms and only to a lesser extent depend on the structure of the previous (internal) electronic layers. In the atoms of all transition elements, the outer electronic layer is formed by two s-electrons*; therefore, the chemical properties of d-elements with increasing atomic number do not change as sharply as the properties of s- and p-elements. All d-elements belong to metals, while filling the outer p-sublevel leads to a transition from a metal to a typical non-metal and, finally, to a noble gas.
After filling the 3d sublevel (n=3, l=2), electrons, in accordance with the second Klechkovsky rule, occupy the 4p sublevel (n=4, l= 1), thereby resuming the construction of the N-layer. This process begins at the gallium atom (Z=31) and ends at the krypton atom (Z=36), the electronic structure of which is expressed by the formula 1s 2 2s 2 2p 6 3s 2 3d 10 4s 2 4p 6. Like the atoms of the previous noble gases - neon and argon, the krypton atom is characterized by the structure of the outer electron layer ns 2np6, where n is the principal quantum number (neon - 2s 2 2p 6, argon - 3s 2 3p 6, krypton - 4s 2 4p 6 ).
Starting with rubidium, the 5s sublevel is filled; this also corresponds to Klechkovsky’s second rule. The rubidium atom (Z=37) has a structure characteristic of alkali metals with one s-electron in the outer electron layer. Thus, the construction of a new - fifth - period of the system of elements begins. In this case, as in the construction of the fourth period, the d-sublevel of the pre-outer electronic layer remains unfilled. Let us recall that in the fourth electronic layer there is already an f-sublevel, the filling of which also does not occur in the fifth period.
In the strontium atom (Z=38), the 5s sublevel is occupied by two electrons, after which the 4d sublevel is filled, so that the next ten elements - from yttrium (Z=39) to cadmium (Z=48) - belong to the transition d-elements. Then from indium to the noble gas xenon there are six p-elements, which complete the fifth period. Thus, the fourth and fifth periods turn out to be quite similar in structure.
* There are d-elements (for example, chromium, molybdenum, elements of the copper subgroup), the atoms of which have only one s-electron in the outer electron layer. The reasons for these deviations from the “normal” order of filling electronic energy sublevels are discussed at the end of the section.
The sixth period, like the previous ones, begins with two s-elements (cesium and barium), which complete the filling of orbitals with a sum (n+l) equal to 6. Now, in accordance with Klechkovsky’s rules, the 4f sublevel (n=4, l=3) with a sum (n+l) equal to 7b and with the smallest possible principal quantum number for this value. In fact, lanthanum (Z=57), located immediately after barium, has not a 4f, but a 5d electron, so its electronic structure corresponds to the formula 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 5d 1 6s 2 . However, already in the element cerium (Z=58), which follows lanthanum, the construction of the 4f sublevel actually begins, to which the only 5d electron present in the lanthanum atom also passes; in accordance with this, the electronic structure of the cerium atom is expressed by the formula 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 2 5s 2 5p 6 6s 2. Thus, the deviation from the second Klechkovsky rule that occurs in lanthanum is temporary: starting from cerium, all orbitals of the 4f sublevel are sequentially filled. Fourteen lanthanides located in this part of the sixth period belong to f-elements and are similar in properties to lanthanum. A characteristic feature of the construction of the electron shells of their atoms is that during the transition to the next f-element, the new electron takes place not in the outer one (n=6) and not in the previous one (n=5), but in an even deeper located, third outer electron layer (n=4).
Due to the absence of significant differences in the structure of the outer and pre-outer electronic layers among lanthanide atoms, all lanthanides exhibit great similarity in chemical properties.
The filling of the 5d sublevel, which began with lanthanum, resumes with hafnium (Z=72) and ends with mercury (Z=80). After this, as in previous periods, six p-elements are located. Here the construction of the 6p sublevel takes place: it begins at thallium (Z=81) and ends at the noble gas radon (Z=86), which ends the sixth period.
The seventh, still unfinished period of the system of elements is built similarly to the sixth. After two s-elements (francium and radium) and one d-element (anemone), there are 14 f-elements, the properties of which show a certain closeness to the properties of actinium. These elements, starting with thorium (Z=90) and ending with element 103, are usually grouped under the general name of actinides. Among them is mendelevium (Z=101), artificially obtained by American physicists in 1955 and named in honor of D.I. Mendeleev. Directly behind the actinides are Kurchatovium (Z=104) and element 105. Both of these elements were artificially obtained by a group of scientists led by Academician G.N. Flerov; they belong to the d-elements and complete the so far known part of the periodic system of elements.
The distribution of electrons across energy levels (layers) in the atoms of all known chemical elements is given in the periodic table of elements located at the beginning of the book.
The sequence of filling energy levels and sublevels in atoms with electrons is schematically presented in Fig. 23, graphically expressing Klechkovsky's rules. Filling occurs from smaller values of the sum (n+l) to larger ones in the order indicated by the arrows. It is easy to see that this sequence coincides with the sequence of filling atomic orbitals shown in Fig. 22.

Rice. 23. Diagram of the sequence of filling electronic energy sublevels in an atom.

Rice. 24. Dependence of the energy of 4f and 5d electrons on the charge of the nucleus Z.
It should be borne in mind that the last scheme (like the Klechkovsky rules themselves) does not reflect the particular features of the electronic structure of the atoms of some elements. For example, when going from a nickel atom (Z=28) to a copper atom (Z=29), the number of 3d electrons increases not by one, but by two at once due to the “leap” of one of the 4s electrons to the 3d sublevel. Thus, the electronic structure of the copper atom is expressed by the formula 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. A similar “leap” of an electron from the outer s- to the d-sublevel of the previous layer also occurs in the atoms of copper analogues - silver and gold. This phenomenon is associated with the increased energy stability of electronic structures corresponding to fully occupied energy sublevels (see § 34). The transition of an electron in a copper atom from the 4s sublevel to the 3d sublevel (and similar transitions in silver and gold atoms) leads to the formation of a completely filled d sublevel and therefore turns out to be energetically favorable.
As will be shown in § 34, electronic configurations with an exactly half-filled sublevel also have increased energy stability (for example, structures containing three p-electrons in the outer layer, five d-electrons in the outer layer, or a network of f-electrons in an even deeper layer). layer). This explains the “leap” of one 4s electron in the chromium atom (Z=24) to the 3d sublevel, as a result of which the chromium atom acquires a stable electronic structure (1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 1) with exactly half filled 3d sublevel; a similar period of the 5s electron to the 4d sublevel occurs in the molybdenum atom (Z = 42).
The above-mentioned violations of the “normal” order of filling energy states in lanthanum atoms (the appearance of a 5d rather than a 4f electron) and cerium (the appearance of two 4f electrons at once) and similar features in the construction of electronic structures of atoms of elements of the seventh period are explained as follows. As the charge of the nucleus increases, the electrostatic attraction to the nucleus of an electron located at a given energy sublevel becomes stronger, and the energy of the electron decreases.
In this case, the energy of electrons located at different sublevels changes differently, since in relation to these electrons the nuclear charge is screened to different degrees. In particular, the energy of 4f electrons decreases with increasing nuclear charge more sharply than the energy of 5d electrons (see Fig. 24). Therefore, it turns out that for lanthanum (Z=57) the energy of 5d electrons is lower, and for cerium (Z=58) it is higher than the energy of 4f electrons. In accordance with this, the electron that was in the 5d sublevel in lanthanum moves to the 4f sublevel in cerium.
| <<< Назад
|
Book: |
Introduction
In 1925, Pauli established the quantum mechanical principle (Pauli exclusion principle).
In any atom there cannot be two electrons that are in the same stationary states, determined by a set of four quantum numbers: n, m, ms.
For example, an energy level can contain no more than two electrons, but with opposite spin directions.
The Pauli principle made it possible to theoretically substantiate Mendeleev's periodic system of elements, create quantum statistics, the modern theory of solids, etc.
Pauli principle
The state of each electron in an atom is characterized by four quantum numbers:
1. Principal quantum number n (n = 1, 2 ...).
2. Orbital (azimuthal) quantum number l (l = 0, 1, 2, ... n-1).
3. Magnetic quantum number m (m = 0, +/-1, +/-2, +/-... +/-l).
4. Spin quantum number ms (ms = +/-1/2).
For one fixed value of the principal quantum number n, there are 2n2 different quantum states of the electron.
One of the laws of quantum mechanics, called the Pauli principle, states:
In the same atom there cannot be two electrons that have the same set of quantum numbers (that is, there cannot be two electrons in the same state).
The Pauli principle provides an explanation for the periodic repetition of the properties of the atom, i.e. Mendeleev's periodic system of elements.
Periodic table of elements by D. I. Mendeleev
In 1869, Mendeleev discovered the periodic law of changes in the chemical and physical properties of elements. He introduced the concept of the serial number of an element and obtained complete periodicity in changes in the chemical properties of elements.
At the same time, some of the cells of the periodic system remained unfilled, because their corresponding elements were unknown at that time. In 1998, the isotope of element 114 was synthesized in Russia.
Mendeleev predicted a number of new elements (scandium, germanium, etc.) and described their chemical properties. Later, these elements were discovered, which completely confirmed the validity of his theory. It was even possible to clarify the values of atomic masses and some properties of elements.
The chemical properties of atoms and a number of their physical properties are explained by the behavior of external (valence) electrons.
Stationary quantum states of an electron in an atom (molecule) are characterized by a set of 4 quantum numbers: principal (n), orbital (l), magnetic (m) and magnetic spin (ms). Each of them characterizes quantization: energy (n), angular momentum (l), projection of angular momentum onto the direction of the external magnetic field (m) and spin projection (ms).
According to the theory, the atomic number of a chemical element Z is equal to the total number of electrons in the atom.
If Z is the number of electrons in an atom that are in a state that is specified by a set of 4 quantum numbers n, l, m, ms, then Z(n, l, m, ms) = 0 or 1.
If Z is the number of electrons in an atom that are in states determined by a set of 3 quantum numbers n, l, m, then Z(n, l, m)=2. Such electrons differ in spin orientation.
If Z is the number of electrons in an atom that are in states determined by 2 quantum numbers n, l, then Z(n, l)=2(2l+1).
If Z is the number of electrons in an atom that are in states determined by the value of the principal quantum number n, then Z(n)=2n2.
Electrons in an atom, occupying a set of states with the same values of the principal quantum number n, form an electronic layer: at n=1 K - layer; at n=2 L - layer; at n=3 M - layer; at n=4 N - layer; at n=5 O - layer, etc.
In each electron layer of an atom, all electrons are distributed among shells. The shell corresponds to a certain value of the orbital quantum number (Table 1 and Fig. 1).
| n | Electronic layer | Number of electrons in shells | Total number of electrons | ||||
| s(l=0) | p(l=1) | d(l=2) | f(l=3) | g(l=4) | |||
| 1 | K | 2 | - | - | - | - | 2 |
| 1 | L | 2 | 6 | - | - | - | 8 |
| 3 | M | 2 | 6 | 10 | - | - | 18 |
| 4 | N | 2 | 6 | 10 | 14 | - | 32 |
| 5 | O | 2 | 6 | 10 | 14 | 18 | 50 |
For a given l, the magnetic quantum number m takes 2l+1 values, and ms takes two values. Therefore, the number of possible states in the electron shell with a given l is equal to 2(2l+1). So the l=0 shell (s - shell) is filled with two electrons; shell l=1 (p - shell) - six electrons; shell l=2 (d - shell) - ten electrons; shell l=3 (f - shell) - fourteen electrons.
The sequence of filling electronic layers and shells in Mendeleev’s periodic system of elements is explained by quantum mechanics and is based on 4 provisions:
1. The total number of electrons in an atom of a given chemical element is equal to the atomic number Z.
2. The state of an electron in an atom is determined by a set of 4 quantum numbers: n, l, m, ms.
3. The distribution of electrons in an atom over energy states must satisfy the minimum energy.
4. The filling of energy states in an atom with electrons should occur in accordance with the Pauli principle.
When considering atoms with large Z, due to an increase in the charge of the nucleus, the electron layer is drawn towards the nucleus and the layer with n=2 begins to fill, etc. For a given n, the state of s-electrons (l=0) is first filled, then p-electrons (l=1), d-electrons (l=2), etc. This leads to periodicity in the chemical and physical properties of elements. For elements of the first period, the shell 1s is filled first; for electrons of the second and third periods - shells 2s, 2p and 3s and 3p.
However, starting from the fourth period (potassium element, Z=19), the sequence of filling the shells is disrupted due to the competition of electrons with similar binding energies. Electrons with larger n, but smaller l (for example, 4s electrons are more tightly bound than 3d) may be more tightly (energetically more favorable) bound.
The distribution of electrons in an atom across shells determines its electronic configuration. To indicate the electronic configuration of an atom, the symbols for filling the electronic states of the shells nl are written in a row, starting with the one closest to the nucleus. The index at the top right indicates the number of electrons in the shell that are in these states. For example, the sodium atom has 2311Na, where Z=11 is the ordinal number of the element in the periodic table; number of electrons in an atom; number of protons in the nucleus; A=23 - mass number (number of protons and neutrons in the nucleus). The electronic configuration has the form: 1s2 2s2 2p6 3s1, i.e. in the layer with n=1 and l=0 - two s-electrons; in the layer with n=2 and l=0 - two s-electrons; in the layer with n=2 and l=1 - six p-electrons; in the layer with n=3 and l=0 - one s-electron.
Along with the normal electronic configuration of the atom, which corresponds to the strongest binding energy of all electrons, excited electronic configurations arise when one or more electrons are excited.
For example, in helium, all energy levels are divided into two level systems: the orthohelium level system, corresponding to the parallel orientation of electron spins, and the parahelium level system, corresponding to the antiparallel spin orientation. The normal configuration of helium 1s2 due to the Pauli principle is possible only with an antiparallel orientation of electron spins, corresponding to parahelium.
Conclusion
So, the Pauli exclusion principle explains, long considered mysterious, the periodic structure of elements discovered by D.I. Mendeleev.
Bibliography
1. Detlaf A.A., Yavorsky B.N. Physics course. - M., 1989.
2. Kompaneets A.S. What is quantum mechanics? - M., 1977.
3. Orir J. Popular physics. - M., 1964.
4. Trofimova T.I. Physics course. - M., 1990.
Has a very significant role in the analysis of microworld phenomena. To determine the state of an electron in a multi-electron atom, the position formulated by W. Pauli is important (. Pauli put forward this assumption even before the advent of quantum mechanics. Pauli formulated it regarding electrons:
There cannot be two electrons in an atom that would be characterized by the same quadruples of quantum numbers $(n,l,m_l,\ m_s)$, that is, more than one electron cannot be in the same state.
Thus, if two electrons have the same principal quantum numbers $(n)$ and the orbital numbers coincide, then their spins should be oriented oppositely (that is, their quantum numbers $m_s\ are equal\ \frac(1)(2)\ and-\ frac(1)(2)$).
Mathematical notation of the Pauli principle
Consider a system of two electrons. If the interaction of electrons is not taken into account, then the wave function of the electron’s motion in space can be considered:
where the indices $a\ and\ b$ denote the states of electrons conventionally numbered $1$ and $2$. The full function for $2$ electrons is the product of the spin wave function and the wave function of their motion in space. We write the spin wave functions as:
Picture 1.
The multiplications result in eight different complete wave functions that have symmetry. In this case we have: the product of two symmetric and two antisymmetric functions gives a symmetric function. Multiplying a symmetric function by an antisymmetric function is an antisymmetric function. As a result, we find that out of eight complete wave functions, $50\%$ are symmetrical:
Antisymmetric functions include:

Figure 2.
Symmetrical functions:

Figure 3.
Not all of the \Psi -- functions written above are possible if you follow the Pauli principle. If the quantum numbers of the two electrons are equal, then the wave function becomes zero. Let's say the electrons make the same motion in their orbits ($a=b$). In this case (according to the Pauli principle), only the opposite orientation of the electron spins is possible. the wave functions that relate to the description of the spin orientation in one direction (8-10) become equal to zero, since the first factor is zero. Wave function (7) is not zero; it describes opposite spins. It turns out that for $a=b$ the antisymmetric wave functions are consistent with the Pauli principle.
Let's consider the second group of wave functions (11-14). When $a=b$, symmetric functions with the same orientation of spins do not become equal to zero. Therefore, they are not acceptable. Function (14) describes the behavior of electrons with spins that are oriented in the opposite direction, which means that it could not be equal to zero. However, when $a=b$ the first factor of the function under consideration is equal to zero, it turns out that the \Psi-function in such cases is always equal to zero, which is incompatible with the Pauli principle, which in this case allows states with different spins. We conclude that symmetric functions are unacceptable.
Based on the above reasoning, we formulate the Pauli principle:
The total wave function of two electrons must be an antisymmetric function with respect to the permutation of electrons. Since formulas (7) - (14) were written without taking into account the interaction of electrons, but in the reasoning we used exclusively the symmetry properties of $\Psi$ - functions that are associated with the identity of electrons and do not depend on their interaction (If we take into account the interaction of electrons, then there is no exchange degeneracy, but the symmetry properties of the wave functions remain, since the identity of the particles is preserved during their interaction.), then all conclusions will apply to interacting electrons.
In case one has to deal with more than $2$ number of electrons, the above statement can be generalized and formulated as:
The wave function of a collection of electrons must be an antisymmetric function with respect to the permutation of any pair of electrons:
Application of the Pauli principle
This principle was used to substantiate Mendeleev’s periodic system and part of the patterns in the spectra.
Thus, the structure of the electronic shells of an atom is based on two principles:
Pauli's principle. It takes into account the quantum properties of possible states of an atom.
The principle of minimum energy: for a given total number of electrons in an atom, a state with minimum energy is realized. This requirement is natural regarding the stability of the atom.
When analyzing the structure of an atom in a first approximation, the interaction energy of electrons is neglected. It is believed that the sum of the energy of an atom is equal to the sum of the energies of the electrons in the field of the nucleus, which is known. This means that it is not difficult to determine the distribution of electrons over different states, taking into account the Pauli principle. The result is a scheme for filling the shells, which, it should be noted, still differs from the real one, but is useful.
Depending on the value of the orbital quantum number $l\ $, the state of an electron in an atom is designated by different letters. Values $l=0,1,2,3,4,5\dots $ are assigned letters $s,p,d,f,g,h$ and in alphabetical order.
The distribution of electrons by state in an atom is written using spectroscopic symbols (Table 1):

Figure 4.
The electronic structure is written as follows: the number on the left is the principal quantum number $(n)$, the spectroscopic symbol itself corresponds to the value of the orbital quantum number $(l)$.
Example 1
Apply the Pauli principle, answer the question: what is the maximum number of electrons $N_(max)$ in an atom that can have the same quantum numbers 1) $n,l,m_l,m_s$; 2) $n$?
Solution:
The state of an electron in an atom is uniquely determined by a set of four quantum numbers:
- main $n\ (n=1,2,3...),$
- orbital$\ l\ (l=0,1,2,...,n-1)$,
- magnetic $m_l$ ($m_l=-l,\dots ,\ -1,0,1,\dots ,l$),
- magnetic spin $m_s$($m_s=\pm \frac(1)(2)$).
1) According to the Pauli principle, one electron in an atom can have a certain set of quantum numbers $n,l,m_l,m_s.$
2) For a given principal quantum number ($n$), the orbital quantum number ($l$) can take values from $0$ to $n-1$, with each value $l$ corresponding to $2l+1$ different values $m_l $, in this case the number of different states that correspond to the known principal quantum number is equal to:
\[\sum\limits^(n-1)_(i=0)(\left(2l+1\right)=n^2).\]
The quantum number $m_s$ can have only two values, which means the maximum number of electrons that have the same principal quantum numbers can be equal to:
Answer: 1) $N_(max)=1$, 2)$\ N_(max)=2n^2.$
Example 2
The electronic layer, characterized by a principal quantum number equal to $n=3$, is completely filled. How many electrons have the same magnetic quantum numbers equal to $m_l=2$?
Solution:
According to answer $2$ of example $1$ we can say that when $n=3$ there can be $18$ electrons in an atom. In this case, $l=0,1,2;;$ $m_l=0,\pm 1,\pm 2;\ m_s=\pm \frac(1)(2)$. It is convenient to summarize the electron distribution in the table (Table 2):

Figure 5.
The table shows that for a pair of quantum numbers $n=3$,$\ m_l=2$ there are two electrons.
Answer: Two electrons.
The history of atomic physics has many ups and downs. But thanks to technological progress, any assumption that arose in the minds of theorists could be tested in laboratory conditions. Since many aspects of the behavior of elementary particles still defy the laws of logic, the scientists who discovered the microworld agreed to accept them “as is,” without explaining the reasons. The Pauli principle refers to the results of those experiments that have not yet found their only explanation.
Controversies in atomic theory
One of the most common successful misconceptions in atomic physics was the planetary atomic model proposed by the English scientist Ernest Rutherford. In the end, it turned out to be not entirely reliable, but it made it possible to draw so many correct conclusions that its benefits were undoubted.
One of the main contradictions of the Rutherford atom was the ability of electrons to radiate. As a result of the loss of energy, any electron would eventually stop moving and fall onto the nucleus. But any atom (except radioactive) is essentially stable, can exist for an indefinitely long time and does not show any signs of self-destruction. To solve this problem, it took the talent of the brilliant Danish physicist Niels Bohr.
Bohr's theory
In 1913, a young unknown physicist from Denmark proposed two changes to be included in classical physics, with the help of which it was possible to explain the facts of observations and make many useful discoveries. Bohr could not explain the reason for the behavior of the electron in orbit, so he based his rules on the “as is” principle. These rules served well in the future and paved the way for new discoveries.
Bohr's rules

The first rule stated that Rutherford's planetary model of the atom was still correct. But the electrons in it move in their orbits without radiation. Bohr's second rule states that electrons can only move in certain “allowed” orbits. For an electron moving along a permitted orbit, the product of momentum and the radius of this orbit is always a multiple of Planck's constant. Thus, electron orbits can only be at those energy levels for which the following rule holds:
(electron momentum * orbital circumference) = n * h,
where h is the plank constant and n is a natural number. Thus, at the smallest allowed orbit, n = 1. The third rule says that the electrons of atoms can be moved (for example, by bombarding them with heavy particles) into a free outer orbit. After this, the electron is able to return to the free inner orbit. In this case, the atom emits excess energy in the form of a quantum of light.
Quantum Limits
Bohr's quantum rule suggests that electrons that are closest to the nucleus have the smallest allowed orbit. At this level, the electron has minimal energy. One would expect that all the electrons in an atom would occupy this orbit and remain in this level. However, this does not happen. The Pauli principle helped explain this contradiction.

Wolfgang Pauli
This famous Austrian physicist was born in Vienna in 1869. At the University of Munich he received an excellent comprehensive education, but devoted all his scientific works to quantum physics. At the age of twenty, Pauli wrote a review article for the Physical Encyclopedia, many pages of which are still relevant today. His scientific works were rarely published; Pauli voiced his most important thoughts and hypotheses in correspondence with his scientific colleagues. The most active correspondence was with N. Bohr and W. Heisenberg. It was the joint work of these three scientists that laid the foundations for modern quantum physics. Based on the experimental data of these three prominent scientists, Pauli formed his principle. For him in 1945, the Austrian scientist received the Nobel Prize.
Electron movement

While studying the motion of the electron, W. Pauli came across many strange aspects in the behavior of this elementary particle. For example, electrons, when moving, behave as if they were rotating around their axis. The electron's own angular momentum is called spin. Two electrons can fit in one place in the orbit, and their spins must be opposite to each other, as the Pauli principle states. The physics of this limitation applies not only to electrons, but also to other particles with a half-integer spin value.
Periodic table and Pauli principle
Chemistry has used the uncertainty principle to explain the internal structure of substances. Now it becomes quite understandable why there are only two elements in the first row of the periodic table. Both hydrogen and helium have at their disposal a single lower orbit, in which there is only one twin place for electrons having opposite spins. The next orbit already contains eight such places. Therefore, eight elements could occupy the second row of the periodic table. This pattern extends to all rows of the periodic table.

Physics of stars
Oddly enough, the laws of behavior of elementary particles extend far beyond the microcosm. For example, stellar physics studies the inner world of aging stars. The Pauli principle also works here, but it is understood a little differently. Now this rule says that in a certain spatial volume it is possible for only two elementary particles with opposite spins to be located. This law is especially clear when observing aging stars. As is known, after an explosion, a supernova rapidly collapses, but not all stars turn into black holes. As the threshold for the maximum density increases (and for an aging star this value is about 10 7 kg/m 3), the internal pressure of the cosmic body begins to grow rapidly. This process has a special scientific term - pressure of degenerate electron gas. Thus, the star stops losing its volume and turns into a small celestial body the size of our Earth. In astrophysics, such stars are called white dwarfs.
Results
The uncertainty principle is one of the first laws of a new type, which differs from all known ideas about the world around us. The new laws are fundamentally different from the rules of classical physics known to us from childhood. If the old rules told us what could happen when carrying out certain actions, then the new type of laws tell us what should not happen.

Algorithms for solving many problems should be built using a slightly modified Pauli principle. By cutting off impossible options for solving problems at the very beginning, there is a chance to find the only correct answer. The practical use of the uncertainty principle significantly reduces the time required for computer processing of information. Previously known only among theoretical physicists, the Pauli principle has long gone beyond the boundaries of quantum physics, thereby identifying new methods for studying the laws of nature.
The Pauli exclusion principle, often called the exclusion principle, limits the number of electrons that can be in one orbital. According to the Pauli principle, any orbital can contain no more than two electrons, and then only if they have opposite spins (unequal spin numbers). Therefore, an atom should not have two electrons with the same four quantum numbers ( n, l, m l , m s).
A lithium atom has three electrons. Lowest energy orbital - 1 s-orbital - can be occupied by only two electrons, and these electrons must have different spins. If we denote spin +1/2 with an arrow pointing up and spin −1/2 with an arrow pointing down, then two electrons with opposite ( antiparallel) spins in the same orbital can be schematically represented as follows:
The third electron in a lithium atom must occupy the orbital next in energy to the lowest orbital, that is, 2 s-orbital.
Hund's rule
Hund's rule (Hund) determines the order in which electrons occupy orbitals that have the same energy. It was derived by the German theoretical physicist F. Hund (Hund) in 1927 based on an analysis of atomic spectra.
According to Hund’s rule, the occupation of orbitals belonging to the same energy sublevel begins with single electrons with parallel (equal sign) spins, and only after single electrons occupy all orbitals can the final occupation of orbitals by pairs of electrons with opposite spins occur . As a result, the total spin (and the sum of spin quantum numbers) of all electrons in the atom will be maximum.
For example, a nitrogen atom has three electrons located on 2 R-sublevel According to Hund's rule, they should be located singly on each of the three 2 R-orbitals. In this case, all three electrons must have parallel spins:
Minimum energy principle
Principle minimum energy determines the order of occupation of atomic orbitals having different energies. According to the minimum energy principle, electrons occupy orbitals with the lowest energy first. The energy of sublevels grows in the series:
1s < 2s < 2 p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f 5d < 6p < 7s < 5f 6d...
A hydrogen atom has one electron, which can be in any orbital. However, in the ground state it should occupy 1 s-orbital having the lowest energy.
In a potassium atom, the last nineteenth electron can occupy either 3 d-, or 4 s-orbital. According to the principle of minimum energy, an electron occupies 4 s-orbital, which is confirmed by experiment.
Note the uncertainty of entry 4 f 5d and 5 f 6d. It turned out that some elements have lower energy 4 f-sublevel, while others have 5 d-sublevel. The same is observed for 5 f- and 6 d-sublevels.
11 Ticket
Periodic law of Mendeleev, a fundamental law that establishes a periodic change in the properties of chemical elements depending on the increase in the charges of the nuclei of their atoms. Opened by D.I. Mendeleev in 1869 when comparing the properties of all elements known at that time and the values of their atomic weights.
The properties of chemical elements, the forms and properties of their compounds are periodically dependent on the magnitude of the charges of the nuclei of their atoms.
The periodic system of chemical elements is a natural classification of chemical elements, which is a tabular expression of the periodic law of D.I. Mendeleev. The prototype of the Periodic Table of Chemical Elements was the table compiled by D.I. Mendeleev March 1, 1869 In 1870 In 1870, Mendeleev called the system natural, and in 1871 - periodic.
The number of elements in the modern Periodic Table is almost twice as large as was known in the 60s of the 19th century. (today - 113), but its structure has not changed much since the time of Mendeleev. Although more than 50 different versions of its image have been published throughout the history of the Periodic Table, the most popular are the short-period and long-period forms proposed by Mendeleev.
The main principle of constructing the Periodic Table is the identification of periods (horizontal rows) and groups (vertical columns) of elements in it. The modern Periodic Table consists of 7 periods (the seventh period must end with the 118th element). The short-period version of the Periodic System contains 8 groups of elements, each of which is conventionally divided into group A (main) and group B (secondary). In the long-period version of the Periodic System there are 18 groups that have the same designations as in the short-period version. Elements of the same group have the same structure of the outer electron shells of their atoms and exhibit a certain chemical similarity.
The group number in the Periodic Table determines the number of valence electrons in the atoms of the elements. At the same time, the groups designated by the letter A contain elements in which settlement is taking place s- and p-sublevels - s-elements (IA- and IIA-groups) and R-elements (IIIA-VIIIA-groups), and in the groups designated by the letter B, there are elements in which d-sublevels - d-elements. Since each major period must contain 10 d-elements (for which five are filled d-orbitals), then the Periodic Table must contain 10 corresponding groups. However, the traditional numbering of groups is only up to eight, so the number of groups d-elements is expanded by introducing additional numbers - these are IB-VIIB, VIIIB0, VIIIB1 and VIIIB2 groups. For f-group number elements are not provided. Usually they are conventionally placed in the cells of the Periodic Table corresponding to lanthanum (lanthanides) and actinium (actinides). The symbols of lanthanides and actinides are moved outside the Periodic Table in the form of separate series.
The period number in the Periodic Table corresponds to the number of energy levels of an atom of a given element filled with electrons.
Period number = Number of energy levels filled by electrons = Designation of last energy level
The order of formation of periods is associated with the gradual population of energy sublevels with electrons. The population sequence is determined by the principle of minimum energy, Pauli's principle and Hund's rule.
The periodic change in the properties of elements in a period is explained by the sequence of filling levels and sublevels in atoms with electrons as the atomic number of the element and the charge of the atomic nucleus increase.

Each element (except f-elements) in the Periodic System correspond to very specific coordinates: period number and group number. Using these coordinates you can not only find an element in the D.I. table. Mendeleev, but also to construct its electronic configuration, taking into account the physical meaning of the meaning of the numbers corresponding to the period and group numbers, as well as the presence of a letter in the group number, which determines the element’s belonging to sections s- And p-elements or d-elements.
Each period begins with the element in whose atom an electron with a given value first appears n(hydrogen or alkaline element), and ends with an element in the atom of which the level with the same n(noble gas). The first period contains only two elements, the second and third - eight each (small periods). Starting from the fourth, periods are called large, since they appear d- And f-elements: the fourth and fifth periods each include 18 elements, the sixth - 32. The seventh period has not yet been completed, but it, like the sixth, should contain 32 elements.
The sequence of occupation of atomic orbitals by electrons can be determined using the rule formulated in 1951 by the Russian agrochemist V.M. Klechkovsky. This rule is often called the "rule" n + l". It reflects the dependence of the energy of atomic orbitals on the principal and orbital quantum numbers.
According to Klechkovsky's rule, the population of energy levels and sublevels in neutral atoms in the ground state by electrons occurs with an increase in the ordinal number of the element in the order of increasing the sum of the main and orbital quantum numbers ( n + l), and with the same value ( n + l) − in order of increasing principal quantum number n.

Klechkovsky's rule has exceptions. In some cases, electrons, without completing complete population s-atomic orbitals may appear on d-orbitals or instead of 4 f-there are 5 atomic orbitals to occupy d-orbitals.
For example, chromium and molybdenum (VIB-group) have 4 s- and 5 s-atomic orbitals, accordingly, have only one electron, and the remaining five fill 3 d- and 4 d-atomic orbitals, since they are half filled d-sublevels have high stability, and the electronic configuration ( n−1)d 5 ns 1 turns out to be more favorable for chromium and molybdenum atoms than (n−1) d 4 ns 2 .
Fully filled is also particularly stable. d-sublevel, therefore, the electronic configuration of the valence electrons of the atoms of copper, silver and gold (IB group) ( n−1)d 10 ns 1 will correspond to a lower energy than ( n−1)d 9 ns 2 .
All elements are divided into four types:
1. In atoms s-elements the s-shells of the outer ns layer are filled. These are the first two elements of each period.
2. In atoms p-elements electrons fill the p-shells of the outer np level. These include the last 6 elements of each period (except the first and seventh).
3. U d-elements the d sublevel of the second outside level (n-1)d is filled with electrons. These are elements of plug-in decades of large periods located between the s- and p-elements.
4. U f-elements the f-sublevel of the third outside level (n-2)f is filled with electrons. These are lanthanides and actinides.
Changes in the acid-base properties of element compounds by groups and periods of the periodic system(Kossel diagram)
To explain the nature of the change in the acid-base properties of compounds of elements, Kossel (Germany, 1923) proposed using a simple scheme based on the assumption that there is a purely ionic bond in the molecules and a Coulomb interaction takes place between the ions. The Kossel scheme describes the acid-base properties of compounds containing E–H and E–O–H bonds, depending on the charge of the nucleus and the radius of the element forming them.
The Kossel diagram for two metal hydroxides (for LiOH and KOH molecules) is shown in Fig. 6.2. As can be seen from the presented diagram, the radius of the Li + ion is smaller than the radius of the K + ion and the OH - group is bound more tightly to the lithium ion than to the potassium ion. As a result, KOH will be easier to dissociate in solution and the basic properties of potassium hydroxide will be more pronounced. The periodic table of elements is a graphic representation of the periodic law and reflects the structure of atoms of elements