The operation of thermal power plants involves the use of large amounts of water. The main part of water (more than 90%) is consumed in cooling systems of various devices: turbine condensers, oil and air coolers, moving mechanisms, etc.
Wastewater is any stream of water removed from a power plant cycle.
Waste or waste water, in addition to water from cooling systems, includes: waste water from hydroash collection systems (HSU), spent solutions after chemical washing of thermal power equipment or its conservation: regeneration and sludge water from water purification (water treatment) plants: oil-contaminated wastewater, solutions and suspensions, arising when washing external heating surfaces, mainly air heaters and water economizers of boilers burning sulfur fuel oil.
The compositions of the listed wastewater are different and are determined by the type of thermal power plant and main equipment, its power, type of fuel, composition of the source water, method of water treatment in the main production and, of course, the level of operation.
Water after cooling the condensers of turbines and air coolers, as a rule, only carries so-called thermal pollution, since its temperature is 8...10 °C higher than the temperature of the water in the water source. In some cases, cooling waters can introduce foreign substances into natural bodies of water. This is due to the fact that the cooling system also includes oil coolers, a violation of the density of which can lead to the penetration of petroleum products (oils) into the cooling water. At fuel oil thermal power plants, wastewater containing fuel oil is generated.
Oils can also enter wastewater from the main building, garages, open switchgears, and oil facilities.
The amount of water in cooling systems is determined mainly by the amount of exhaust steam entering the turbine condensers. Consequently, most of this water is at condensing thermal power plants (CHPs) and nuclear power plants, where the amount of water (t/h) cooling the turbine condensers can be found by the formula Q = KW where W is the power of the station, MW; K-factor, for thermal power plants K = 100...150: for nuclear power plants 150...200.
In power plants using solid fuels, removal of significant quantities of ash and slag is usually carried out hydraulically, which requires large quantities of water. At a thermal power plant with a capacity of 4000 MW, operating on Ekibastuz coal, up to 4000 t/h of this fuel is burned, which produces about 1600...1700 t/h of ash. To evacuate this amount from the station, at least 8000 m3/h of water is required. Therefore, the main direction in this area is the creation of circulating gas recovery systems, when clarified water freed from ash and slag is sent back to the thermal power plant into the gas recovery system.
The waste waters of gas treatment facilities are significantly contaminated with suspended substances, have increased mineralization and, in most cases, increased alkalinity. In addition, they may contain compounds of fluorine, arsenic, mercury, and vanadium.
Effluents after chemical washing or conservation of thermal power equipment are very diverse in composition due to the abundance of washing solutions. For washing, hydrochloric, sulfuric, hydrofluoric, sulfamic mineral acids are used, as well as organic acids: citric, orthophthalic, adipic, oxalic, formic, acetic, etc. Along with them, Trilon B, various corrosion inhibitors, surfactants, thiourea, hydrazine, nitrites, ammonia.
More articles on the topic
Ecology of water bodies
Water is the most valuable natural resource. It plays an exceptional role in metabolic processes that form the basis of life. Water is of great importance in industrial and agricultural production; the need for e...
Monitoring and audit of industrial and environmental safety
The transition to new management mechanisms and a developed market is impossible without rational and efficient use of resources, reducing environmental and economic damage from accidents and injuries. Solving this important problem requires...
Contaminated wastewater from thermal power plants and their water treatment plants consists of streams of different quantity and quality. They include (in descending order of quantity):
a) wastewater from both circulating and direct-flow (open) hydroash and slag removal systems (HSU) of power plants operating on solid fuels;
b) blowdown water from circulating water supply systems of thermal power plants, discharged continuously;
c) wastewater from water treatment plants (WTP) and condensate treatment plants (CPU), discharged periodically, including: fresh, sludge-contaminated, saline, acidic, alkaline, oily and oil-contaminated waters of the main building, fuel oil and transformer facilities of thermal power plants;
d) blowdown water from steam boilers, evaporators and steam converters, discharged continuously;
e) oily and slushy snow and rain runoff from the territory of the thermal power plant;
f) washing water from RAH and heating surfaces of boilers (wastewater from RAH boilers operating on fuel oil is discharged 1-2 times a month or less, and from other surfaces and when burning solid fuels - more often);
g) oily, contaminated external condensates, suitable after their cleaning for feeding steam evaporator boilers;
h) waste, spent, concentrated, washing acidic and alkaline solutions and wash water after chemical washing and conservation of steam boilers, condensers, heaters and other equipment (discharged several times a year, usually in summer);
i) water after hydraulic cleaning of fuel shops and other premises of thermal power plants (usually discharged once a day per shift, more often during the day).
Relationship between fresh and waste water from power plants
At thermal power plants there must be a unified water supply and drainage system, in which waste water of the same type, directly or after some treatment, could be the source for other consumers of the same thermal power plant (or external ones). For example, waste waters of direct-flow water supply systems after condensers, as well as blowdown waters of circulating systems with a small (1.3-1.5 times) evaporation, as well as oil-contaminated wastewater from thermal power plants can be the source water of the water treatment plant, as well as the last portions washing water from desalting filters.
All waste water returned to the “head” of the process should not need to be treated with reagents during pre-treatment; if it is necessary to treat with lime, soda and coagulant, they should be mixed (averaged) in a collecting tank. The capacity of this tank should be designed to collect 50% of all wastewater from the water treatment unit per day, including 30% of the wastewater from the ion exchange part. It is not advisable to mix clear soft and sludge waste water. It should be taken into account that at least 50% of all waste water of the water treatment plant, including all waste water of pre-treatment of all types, including waste water after loosening ion exchange filters with fresh water, the last portions of washing water of ion exchange filters of desalting plants, as well as water discharged when emptying clarification plants and ion exchange filters, have salt content, hardness, alkalinity and other indicators that are the same or even better than pre-purified and, especially, source water, and therefore can be returned to the “head” of the process, to clarifiers, or, even better, without additional treatment with reagents. for clarification, H- or Na-cation exchange filters.
In addition to a single common sewerage system for all types of fresh water, there must also be separate discharge channels for saline and acidic waters (alkaline waters must be completely used in the cycle, including for neutralization). This water must be collected in special pit tanks.
Due to the periodic operation of earth pits (mainly in the summer) for cleaning solutions and boiler wash waters after chemical washes, after installations for neutralizing these waters and wash waters, the RVP should provide the possibility of supplying various discharged acidic, alkaline and saline waters of the WPU to these structures for joint or alternate neutralization, settling, oxidation and transferring them to the gas storage system or other consumers. When obtaining vanadium oxide from RVP wash waters, these waters are not mixed with others before the vanadium is separated. In this case, the neutralized installation or, at least, its pumps and fittings must be located in an insulated room.
Saline waters after Na-cation exchange filters are divided into three parts according to their quality and used in different ways.
A concentrated spent salt solution containing 60-80% of removed hardness with a 50-100% excess of salt and constituting 20-30% of the total volume of saline water should be sent to the gas treatment system or for softening with return to the water treatment plant, or for evaporation to obtain solid salts Ca, Mg, Na, CI, S0 4, or into earthen pits, from where, after mixing with other wastewater, dilution and joint neutralization, it can be sent to the sewer system, for the needs of thermal power plants or external consumers. The second part of the spent solution, containing 20-30% of the total hardness removed with a 200-1000% excess of salt, should be collected in a tank for reuse. The third and last part - washing water - is collected in another tank for use during loosening, if it cannot yet be sent to the “head” of the process or for the first stage of washing.
Concentrated saline water after Na-cation exchange filters and neutralized water from N-cation exchange and anion exchange filters (the first portions) can be supplied to gas treatment systems for transporting ash and slag. The accumulation of gas compounds Ca(OH) 2 and CaS0 4 in water leads to saturation and supersaturation of water with these compounds, releasing them in solid form on the walls of pipes and equipment. Oils and petroleum products from wastewater remaining in it after oil traps are sorbed by ash and slag when discharged into the gas treatment system. However, with a high content of petroleum products, they may not be completely sorbed and may be present in ash dumps in the form of floating films. To prevent them from entering with the discharged water into public water bodies, receiving wells for discharge water with gates (“pans”) are built at ash dumps to retain floating oil products.
Soft alkaline, sometimes hot, blowdown waters of steam boilers, evaporators, steam converters after using their vapor and heat, as well as soft alkaline washout waters of anion exchange filters can serve as feed water for less demanding steam boilers, and also (in the absence of heat exchangers with brass pipes in the heating system) make-up water for closed heating systems. If they contain Na 3 P0 4 phosphates in an amount of more than 50% of the total salt content, they can be used for stabilization treatment of circulating water, as well as for dissolving salt in order to soften its solution with alkalis and phosphates contained in the blowing water.
When choosing a method for treating saline, acidic or alkaline waters after regeneration of ion exchange filters, sharp fluctuations in the concentrations of soluble substances in these waters should be taken into account: maximum concentrations in the first 10-20% of the total volume of discharged water (the actual waste solutions) and minimum concentrations in the last 60-80 % (washing water). The same concentration fluctuations are observed in waste solutions and wash waters after chemical washes of steam and hot water boilers and other apparatus.
While wash waters with a small concentration of soluble substances can be relatively easily neutralized (mutually), oxidized and generally purified from removable contaminants, purification of a large volume of a more concentrated mixture of waste solutions and wash waters requires large amounts of equipment, significant labor costs, funds and time.
Spent alkaline solutions and wash waters after regeneration of anion exchange filters (except for the first portion of solution after 1st degree filters) must be reused inside the water supply unit. The first portion is sent to neutralize acidic waste waters of water treatment plants and thermal power plants.
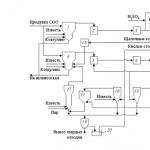
Scheme of a drainless thermal power plant
In Fig. 13.18 shows as an example a drainless water supply scheme for a coal-fired thermal power plant. Ash and slag from the boilers are supplied to ash dump 1. Clarified water 2 from the ash dump is returned to the boilers. If necessary, part of this water is purified at a local treatment plant 3. The resulting solid waste 4 is supplied to the ash dump 1. Partially dehydrated ash and slag are disposed of. Dry ash removal is also possible, which simplifies the disposal of ash and slag.
Flue gases from 5 boilers are purified in gas desulfurization unit 6. The resulting wastewater is purified using technology using reagents (lime, polyelectrolytes). Purified water is returned to the gas purification system, and the resulting gypsum sludge is transported for processing.
Wastewater 7 generated during chemical washing, preservation of equipment and washing of convective heating surfaces of boilers is supplied to the appropriate treatment units 8, where it is processed using reagents using one of the previously described technologies. The main part of purified water 9 is reused. Vanadium containing sludge 10 is transported for disposal. Sludge 11 formed during wastewater treatment, together with part of the water, is supplied to the ash dump 1 or stored in special sludge storage tanks. At the same time, as the operating experience of Saransk CHPP-2 has shown, when boilers are fed with distillate distillate, operational cleaning of the boilers is practically not necessary. Consequently, wastewater of this type will be practically absent or its amount will be insignificant. Water from equipment conservation is disposed of in a similar way, or conservation methods are used that are not accompanied by the generation of wastewater. After neutralization, part of this wastewater can be uniformly supplied to the water treatment facility for processing together with the purge waters of the 12 SOO (recirculation cooling system).
The source water is supplied directly or after appropriate treatment at the water treatment plant to the SOO. The need for treatment and its type depend on the specific operating conditions of the thermal power plant, including the composition of the source water, the required degree of its evaporation in the coolant, the type of cooling tower, etc. In order to reduce water losses in the cooler, cooling towers can be equipped with drop eliminators or semi-dry or dry cooling towers can be used . Auxiliary equipment 13, the cooling of which may contaminate the circulating water with petroleum products and oils, is separated into an independent system. The water of this system is subjected to local purification from petroleum products and oil in node 14 and is cooled in heat exchangers 15 by water 16 from the main COO cooling circuit of the turbine condensers. Part of this water 17 is used to replenish losses in the cooling circuit of auxiliary equipment 13. Oil and petroleum products 18 separated in unit 14 are fed into boilers for combustion.
Part of the water 12, heated in the heat exchangers 15, is sent to the VPU, and its excess 19 is sent for cooling in the cooling tower.
Blowing water 12 SOO is processed at a water treatment facility using technology using reagents. Part of the softened water 20 is supplied to make up the closed heating network in front of the heating water heaters 21 of the network water. If necessary, part of the softened water can be returned to the SOO. The required amount of softened water 22 is sent to the MIU. Blowdowns from 23 boilers are also supplied here, as well as condensate 24 from the fuel oil facility directly or after cleaning in unit 25. Oil products 18 separated from the condensate are burned in boilers.
Steam 26 of the first stage of the MIU is supplied to production and to the fuel oil facility, and the resulting distillate 27 is supplied to feed the boilers. Condensate from production and condensate from network heaters 21 after treatment in a condensate treatment unit (CP) are also supplied here. Wastewater from 28 KO and the block desalting plant BOU is used in the water treatment plant. Blowing water 29 MIU is also supplied here to prepare the regeneration solution according to the previously described technology.
Stormwater from the territory of the thermal power plant is collected in the stormwater storage tank 30 and, after local treatment at node 31, is also supplied to the SOO or to the water treatment facility. Oil and oil products 18 separated from water are burned in boilers. Groundwater can also be supplied to the SWS without or after appropriate treatment.
When working using the described technology, lime and gypsum sludge will be formed in significant quantities.
There are two promising directions for creating drainless thermal power plants:
Development and implementation of economical and environmentally advanced innovative technologies for the preparation of additional water for steam generators and make-up water for heating networks;
Development and implementation of innovative nanotechnologies for the most complete processing and disposal of generated wastewater with the production and reuse of initial chemical reagents in the station cycle.
Figure 13. Scheme of thermal power plants with high environmental performance
Abroad (especially in the USA), due to the fact that a license to operate a power plant is often issued under the condition of complete drainage, water treatment and wastewater treatment schemes are interconnected and represent a combination of membrane methods, ion-exchange and thermal desalination. For example, the water treatment technology at the North Lake power plant (Texas, USA) includes two parallel operating systems: coagulation with ferrous sulfate, multilayer filtration, then reverse osmosis, double ion exchange, mixed layer ion exchange or electrodialysis, double ion exchange, ion exchange in a mixed layer.
Water treatment at the Braidwood nuclear station (Illinois, USA) involves coagulation in the presence of a chlorinating agent, lime milk and flocculant, filtration on sand or active carbon filters, ultrafiltration, electrodialysis, reverse osmosis, cation exchange layer, anion exchange layer, mixed layer.
An analysis of the technologies implemented for the processing of highly mineralized wastewater at domestic power plants allows us to assert that complete recycling is feasible only through evaporation in various types of evaporation plants. At the same time, clarifier sludge (mainly calcium carbonate), gypsum-based sludge (mainly calcium sulfate dihydrate), sodium chloride, sodium sulfate are obtained as products suitable for further sale.
At the Kazan CHPP-3, a closed cycle of water consumption was created through the complex processing of highly mineralized wastewater from the thermal desalting complex to produce a regeneration solution and gypsum in the form of a commercial product. When operating according to this scheme, an excess amount of evaporation unit purge water is generated in a volume of about 1 m³/h. The purge is a concentrated solution that mainly contains sodium cations and sulfate ions.

Figure 14. Technology for processing wastewater from the thermal desalting complex of Kazan CHPP-3.
1, 4 – clarifiers; 2, 5 – clarified water tanks; 3, 6 – mechanical filters; 7 – sodium cation exchange filters; 8 – tank, chemically purified water; 9 – chemically purified water to make up the heating network; 10 – concentrate tank of the evaporation unit; 11 – reactor tank; 12, 13 – tanks for various purposes; 14 – tank of clarified solution for regeneration (after acidification and filtration) of sodium cation exchange filters; 15 – crystallizer; 16 – crystallizer-neutralizer; 17 – thermochemical softener; 19 – bunker; 20 – pit; 21 – excess evaporator purge; 22 – filter with active carbon loading; 23 – electric membrane unit (EMU).
An innovative nanotechnology has been developed for processing excess purge water of a thermal desalting complex based on an electric membrane installation to produce alkali and softened water. The essence of the electromembrane method is the directed transfer of dissociated ions (salts dissolved in water) under the influence of an electric field through selectively permeable ion-exchange membranes.
The operation of thermal power plants involves the use of large amounts of water. The main part of water (more than 90%) is consumed in cooling systems of various devices: turbine condensers, oil and air coolers, moving mechanisms, etc.
Wastewater is any stream of water removed from a power plant cycle.
Waste or waste water, in addition to water from cooling systems, includes: waste water from hydroash collection systems (HSU), spent solutions after chemical washing of thermal power equipment or its conservation: regeneration and sludge water from water purification (water treatment) plants: oil-contaminated wastewater, solutions and suspensions, arising when washing external heating surfaces, mainly air heaters and water economizers of boilers burning sulfur fuel oil.
The compositions of the listed wastewater are different and are determined by the type of thermal power plant and main equipment, its power, type of fuel, composition of the source water, method of water treatment in the main production and, of course, the level of operation.
Water after cooling the condensers of turbines and air coolers, as a rule, only carries so-called thermal pollution, since its temperature is 8...10 °C higher than the temperature of the water in the water source. In some cases, cooling waters can introduce foreign substances into natural bodies of water. This is due to the fact that the cooling system also includes oil coolers, a violation of the density of which can lead to the penetration of petroleum products (oils) into the cooling water. At fuel oil thermal power plants, wastewater containing fuel oil is generated.
Oils can also enter wastewater from the main building, garages, open switchgears, and oil facilities.
The amount of water in cooling systems is determined mainly by the amount of exhaust steam entering the turbine condensers. Consequently, most of this water is at condensing thermal power plants (CHPs) and nuclear power plants, where the amount of water (t/h) cooling turbine condensers can be found by the formula Q=KW Where W- station power, MW; TO-coefficient for thermal power plants TO= 100...150: for nuclear power plants 150...200.
In power plants using solid fuels, removal of significant quantities of ash and slag is usually carried out hydraulically, which requires large quantities of water. At a thermal power plant with a capacity of 4000 MW, operating on Ekibastuz coal, up to 4000 t/h of this fuel is burned, which produces about 1600...1700 t/h of ash. To evacuate this amount from the station, at least 8000 m 3 /h of water is required. Therefore, the main direction in this area is the creation of circulating gas recovery systems, when clarified water freed from ash and slag is sent back to the thermal power plant into the gas recovery system.
The waste waters of gas treatment facilities are significantly contaminated with suspended substances, have increased mineralization and, in most cases, increased alkalinity. In addition, they may contain compounds of fluorine, arsenic, mercury, and vanadium.
Effluents after chemical washing or conservation of thermal power equipment are very diverse in composition due to the abundance of washing solutions. For washing, hydrochloric, sulfuric, hydrofluoric, sulfamic mineral acids are used, as well as organic acids: citric, orthophthalic, adipic, oxalic, formic, acetic, etc. Along with them, Trilon B, various corrosion inhibitors, surfactants, thiourea, hydrazine, nitrites, ammonia.
As a result of chemical reactions in the process of washing or preserving equipment, various organic and inorganic acids, alkalis, nitrates, ammonium salts, iron, copper, Trilon B, inhibitors, hydrazine, fluorine, methenamine, captax, etc. can be discharged. Such a variety of chemicals requires an individual solution for the neutralization and disposal of toxic waste from chemical washes.
Water from washing external heating surfaces is formed only at thermal power plants that use sulfur fuel oil as the main fuel. It should be borne in mind that the neutralization of these washing solutions is accompanied by the production of sludge containing valuable substances - vanadium and nickel compounds.
During the operation of water treatment of demineralized water at thermal power plants and nuclear power plants, wastewater arises from the storage of reagents, washing of mechanical filters, removal of sludge water from clarifiers, and regeneration of ion exchange filters. These waters carry significant amounts of calcium, magnesium, sodium, aluminum, and iron salts. For example, at a thermal power plant with a chemical water treatment capacity of 2000 t/h, salts are discharged up to 2.5 t/h.
Non-toxic sediments are discharged from pre-treatment (mechanical filters and clarifiers) - calcium carbonate, iron and aluminum hydroxide, silicic acid, organic substances, clay particles.
And finally, at power plants that use fire-resistant liquids such as IVVIOL or OMTI in the lubrication and control systems of steam turbines, a small amount of wastewater contaminated with this substance is generated.
The main regulatory document establishing the system for the protection of surface waters is the “Rules for the protection of surface waters (standard regulations)” (Moscow: Goskomprirody, 1991).
Heated wastewater from thermal power plants and other industries
cause “thermal pollution”, which threatens quite serious
consequences: there is less oxygen in heated water, the thermal regime changes sharply, which negatively affects the flora and fauna of reservoirs, while favorable conditions arise for the massive development of blue-green algae in reservoirs - the so-called “water bloom”.
When used in technological processes, water becomes contaminated with various organic and mineral substances, including toxic ones. One of the sources of environmental pollution with harmful substances, and primarily heavy metals, is wastewater from electroplating industries.
Calculation of characteristics of wastewater discharges from enterprises into water bodies
The technological cycle of one of the industrial enterprises requires the consumption of significant quantities of water. The source is usually a river located near the enterprise. Having gone through the technological cycle, the water is almost completely returned to the river in the form of wastewater from an industrial enterprise. Depending on the profile of the enterprise, wastewater may contain a variety of chemical components that are harmful in terms of sanitary and toxicological characteristics. Their concentration, as a rule, is many times higher than the concentration of these components in the river. At some distance from the wastewater discharge site, water
rivers are taken for the needs of local water use of a very different nature
(for example, household, agricultural). The problem requires calculating
the concentration of the most harmful component after diluting the wastewater of the enterprise with river water at the place of water use and monitor the change in this concentration along the river fairway. And also determine the maximum permissible runoff (MAF) for a given component in the runoff. Characteristics of the river: flow speed - V, average depth in the area - H, distance to the place of water use - L, water flow in the river - Q1; step with which it is necessary to trace the change in the concentration of the toxic component along the river fairway - LS.
Characteristics of the flow: harmful component, water flow -Q2, concentration
harmful component - C, background concentration - Sf, maximum permissible concentration - MAC.
Options for calculating the characteristics of wastewater discharges from enterprises into water bodies:
ε=1; LФ/Lpr=1
SOLUTION:
Many factors: the state of the river, banks and sewage affect the speed
movement of water masses and determine the distance from the place of waste discharge
water (SW) to the point of complete mixing.
TO= γ-Ql+Q2
where y is the coefficient, the degree of completeness of wastewater in the reservoir.
The conditions for the discharge of wastewater into a reservoir are usually assessed taking into account their influence on
the nearest point of water use where the dilution factor should be determined.
The calculation is carried out using the formulas:
1- β
at= (Q1/ Q2) β
β = exp( -α * ),
Where α -coefficient taking into account hydrological mixing factors.
L is the distance to the water intake site.
α = ε·(Lф/ Lnp) · ,
Where ε -coefficient depending on the location of discharge into the river. ε =1, upon release
near the shore.
Lf/Lpr is the coefficient of river tortuosity, equal to the ratio of the distance along the fairway of the full length of the channel from the outlet of the water supply to the place of the nearest water intake to the distance between these two points in a straight line.
Based on the fact that in this problem it is assumed that the rivers under study are flat, we will find the D-coefficient of turbulent diffusion,
D= V*H = 1 0.9= 0,0045
where V is the average current speed, m/s;
H - average depth, m.
Knowing D, we find:
γ=
![]()
So, the real dilution factor is:
K= 0,025*40+0,7 =2428
The actual concentration of a harmful component in a reservoir at the location of the nearest
water intake is calculated by the formula:
St.= (WITH -Sf) = 0.5 - 0.001 = 0.2
K 2.428
0.2 > 0.01, this means that this value exceeds the maximum permissible concentration
It is also necessary to determine how much pollutants can
be reset by the enterprise so as not to exceed the standards. Calculations are carried out only for conservative substances according to the sanitary and toxicological indicator of harmfulness. The calculation is carried out according to the formula:
From Art.pred. = K·(MPC - C f) + MAC=2.428(0.01-0.001)+0.01=0.032 mg/l=0.000032.mg/m 3
where C st. limit is the maximum (limit) concentration that can be
allowed in the SV, or that level of SV purification at which, after mixing them with
water in the reservoir at the first (calculation) point of water use, degree of pollution
does not exceed the maximum permissible concentration.
The maximum permissible flow MAP is calculated using the formula:
MDS = C st.pred ·Q2 = 0.000032 ·0.7 = 2.24·10-5 mg/s
Let's plot the distribution of the concentration of a harmful component
Depending on the distance to the place of discharge of SW along the river bed with a step of LS=15 m, SW=f(L):
 |
Conclusions: Having solved this problem, we obtained the real concentration of the harmful component in the reservoir at the location of the nearest water intake, St = 0.2, it turned out to be more than the maximum permissible concentration of harmful substances in the reservoir, which means that the reservoir is very polluted and requires immediate cleaning, and the enterprise that discharges its wastewater into it must be checked for sanitary standards.
List of used literature:
1) Podobedov N.s. Natural resources of the Earth and environmental protection.
M, Nedra, 1985.
2) Sladkopevtsev With": Environmental management systems. M, MNEPU, 1998.
Z) Arustamov E. A. et al. Environmental management: Textbook. - 7th ed. reworked And additional - M.: Publishing and trading corporation "Dashkov I Co", 2005.
4) Gurova T.F., Fundamentals of ecology and environmental management: Textbook.
allowance / T. F. Gurova, L. V. Nazarenko. - M.: Onyx Publishing House, 2005.
5) Zelenov V.A. Fundamentals of environmental economics and environmental protection
environment. Uch. manual for universities. - Yaroslavl, 1987.
Wastewater from different sources is treated using appropriate methods.
· From thermal power cooling systems
equipment
Recirculating cooling systems are used: with cooling towers,
with spray devices, with a cooling pond. With the introduction of circulating cooling systems, water quality deteriorates in the process of evaporation and droplet entrainment, which significantly worsens the technical and economic performance of thermal power equipment.
To combat biological fouling and mineral deposits in condenser tubes, the following methods are used: mechanical (rubber balls circulating in condenser tubes); electromagnetic water treatment; chemical (acidification, decarbonization, treatment with phosphates - OEDPA, chlorine, etc.).
A method is used to maintain an optimal salt balance in the system, directing the blowdown water from cooling towers to the water pumping station for the preparation of make-up water for the heating network (this option is used at many thermal power plants).
Biological control methods include, in particular, breeding herbivorous fish in water bodies (in a system with cooling ponds). If no other types of wastewater are discharged into the cooling systems, then practically from a chemical point of view they do not threaten water bodies. However, it should be said that cooling systems usually also include turbine oil coolers, which often results in oil flowing into the cooling water, which then ends up in water bodies. Recently, reliable plate oil coolers have been used, which have eliminated this problem.
· From water treatment and condensate treatment
From an economic point of view, the main direction to reduce the amount of salts discharged from water treatment plants is the use of modern water treatment technologies with reduced reagent costs.
When treating water treatment plant wastewater, two groups of wastewater should be distinguished: discharges from pre-treatment plants and discharges from desalination plants.
Pre-treatment methods are organically included in existing water treatment systems and should retain their importance in the near future. An important advantage of pre-treatment over other methods, from the point of view of protecting water bodies, is that the discharged impurities are in the water in the form of sediment. This makes it much easier to separate them from the water.
The most preferable schemes for treating purge water with clarifiers are those in which the clarified purge water can be returned back to the air intake unit. From the point of view of reducing the size of the areas occupied by the sludge neutralization and disposal unit, the most interesting is the scheme with the return of blowing water to the air intake unit without its neutralization and with sludge dewatering using press filters or drum-vacuum filters. In this case, the maximum possible amount of clarified water from all options can be returned to the water treatment facility, and therefore, the possible consumption of reagents during pre-treatment and the amount of discharged impurities (in particular, in the form of sludge) will be minimal. In this case, the area required for organizing a sludge dump is also significantly reduced. In Russia, at one time, pilot tests were carried out on burning clarifier sludge in submersible combustion apparatuses and obtaining lime from it again, which can again be used in the VPU scheme. This method has not been widely used for economic reasons. Currently, as a rule, the blowdown water is subjected to settling, after which the clarified water is returned to the cycle, and the concentrated sludge with part of the water is sent through the gas treatment system to the ash dump.
Apart from a certain amount of coarse impurities entering the wastewater from the desalting part of the water treatment unit during loosening of the filters, these waters are true solutions of salts, which greatly complicates the task of their treatment. This also applies to purge water from evaporators and steam converters.
Currently, depending on local conditions, such wastewater is recommended to be directed: 1) to reservoirs in compliance with sanitary, hygienic and fishery requirements for the quality of water in the reservoir in the design solution; 2) into the hydraulic ash removal system using both ash and sludge for the needs of hydraulic transport; 3) into evaporation ponds under favorable climatic conditions; 4) for evaporation plants; 5) into underground aquifers that are not suitable for economic purposes and are reliably isolated from groundwater used for water supply. Wash water from electromagnetic filters is discharged into ash and sludge dumps.
When discharging sewage water from a water treatment facility, one should take into account its sharply variable flow rate and significant fluctuations in pH values. Therefore, it is recommended to collect wastewater from the water treatment plant in special storage tanks. The capacity of such tanks must be selected taking into account the filter regeneration cycles. When discharging wastewater from the water treatment plant in the hydroash removal system (GSU), these waters should not change the composition of the water circulating in the system, i.e. do not lead to deposits.
However, the most widespread process is neutralization with lime milk, since in this case the salt content does not increase as sharply as when using other reagents. This is explained by the fact that neutralization with lime is accompanied by the formation of sediment, which can be removed from the water.
The technological process of neutralization consists of filling neutralizing tanks with acidic and alkaline waters, supplying a certain amount of neutralizing reagent and mixing the liquid in the tank until a constant pH value of the neutralized water is established.
To reduce emissions, loosening, regeneration and flushing waters are reused at the water treatment plant. However, it is possible to significantly reduce discharges only if modern water treatment technologies are used (countercurrent and double-flow countercurrent ionization schemes), which make it possible to reduce the consumption of reagents (acids and alkalis) to 1.5 stoichiometries in relation to the amount of retained salts. These technologies in various modifications have been widely used abroad for a long time and are increasingly being used in Russia. A desalting plant using this technology has been in operation for a long time at the Volzhskaya CHPP-2, while the specific consumption of reagents is 1.7...1.8 g-eq./g-eq.
Membrane technologies for water desalination (electrodialysis and reverse osmosis) differ significantly from chemical desalination. In this case, desalting occurs practically without the use of reagents, only through ion-exchange membranes, i.e. The same amount of salts that were taken from it with water are returned to nature, but only in a more concentrated form (in less water). It must be borne in mind that membrane water purification technologies are economically feasible, as a rule, when the quality of the source water is low, 2...4 times worse than average water. A reverse osmosis unit (ROU) with a capacity of 50 m3/h is in operation at the Voronezh CHPP. Preliminary purification of water before supplying it to the treatment facility is carried out through pre-treatment (coagulation with liming and removal of suspended matter on mechanical filters) and subsequent softening on Na-cation filters. A single-stage electrodialysis unit (UEO-100-4/25) with a capacity of 100 m3/h made it possible, for example, to reduce the salt content in water by 75%. The schematic diagram of a chemical treatment plant based on electrodialysis units is based on the principle: pre-treatment; post-treatment using fine filters; desalting in electrodialysis units; post-treatment using ion exchange filters and FSD.
The method of preparing additional water for steam boilers using evaporators has found wide application in the energy sector (both in Russia and abroad). The most promising and optimal from an economic point of view are flash evaporators (IEV). Before supplying water to the evaporators, the same pre-cleaning is required as for the UOO.
The oxygen water-chemical regime currently used in almost all Russian power plants with direct-flow boilers makes it possible to increase the filtration cycle of condensate purification filters (CPF) by 3...5 times, thereby reducing discharges into the environment by the same amount.
· from petroleum products
Sedimentation is the most common method for separating petroleum products from wastewater from various enterprises. The main reasons for this are spontaneity, cost-effectiveness of the process and the seemingly obvious simplicity of calculation and design of settling structures.
Flotation of dispersed particles from wastewater is based on their ability to attach to a hydrophobic surface immersed in water. The surface of gas bubbles with which the treated liquid is previously saturated is usually used as such a surface. Bubbles that float or form in the liquid volume capture particles and transport them to the surface, from where the particles are removed as a concentrate.
Water is saturated with air in pressure flotation units by dissolving it under pressure in pressure tanks. Waste water is taken from the storage tank by a pump and supplied to the pressure tank. An air ejector is installed on the water recirculation line from the pressure pipe of the pump to the suction pipe, supplying air in a volume of 3...5\% of the water flow through the pump. The steam-air mixture compressed in the pump is kept in a pressure tank for 3...5 minutes, after which it is fed through throttling valves into the flotation tank, where the bubbles, passing through a layer of water, float oil particles.
The average efficiency of water purification according to the pressure flotation scheme in such flotation settlers at a pressure in the pressure tank of 4.0...4.5 kgf/cm2 and using coagulation is about 88%.
Filtration is usually used at the final stages of wastewater treatment and on this basis it is often classified as a post-treatment method. However, the filtration method can be successfully used as the main one if the concentration of petroleum products in the wastewater supplied for treatment does not exceed 10...20 mg/dm3.
The process of filtering wastewater contaminated with petroleum products is based on the adhesion (sticking) of emulsified drops of petroleum products to the surface of the grains of the filter material. In general, the filtration process is determined by many technological parameters, primarily the properties of the porous and filtered media, hydrodynamic process conditions and temperature.
During filtration, oil particles are trapped in a layer, filling part of the pore volume and saturating this volume. An increase in saturation leads to the fact that the filter material is not able to retain the trapped oil and it flows in the form of a film along the walls of the layer channel in the direction of flow. At some point in time, in the cross section of the layer, an equilibrium is established between the amount of oil released from the flow onto the surface of the layer and the amount of oil flowing from this volume in the form of a film into deeper layers. In this case, the concentration reaches a critical value, which can be considered the maximum saturation of the layer with oil under the given conditions of the filtration process. Over time, the front of maximum saturation shifts to the lower boundary of the layer and the oil concentration in the filtrate increases. This serves as a signal to turn off the filter for regeneration if it does not turn off due to a difference in water pressure.
The schemes of treatment facilities for thermal power plants more or less fully present the methods described above for purifying water from oil products. Wastewater contaminated with petroleum products is collected in a balancing tank, usually designed for a two-hour capacity of the structures.
In the tank, the primary settling of coarse petroleum products and sinking impurities (sand, corrosion products, etc.) occurs. Removal of floating oil products is carried out through a funnel installed on the float, and settled impurities are removed through a pipe in the lower part of the tank. After the initial settling, the wastewater is sent to an oil trap. The water purified in the oil trap is drained into an intermediate tank and pumped into a pressure flotation unit, after which it is purified in two stages of filtration. Typically, filters loaded with anthracite are used as the first stage. In the second stage, purification is carried out using activated carbon filters. Contaminated filters are washed with hot water and discharged into a homogenizing tank.
The absorption capacity of petroleum products, g/g, for various brands of activated carbon is on average: AG-5 – 0.15; AG-3 – 0.08; AP-3 – 0.06; BAU – 0.04; Berezovsky - 0.03. As you can see, AG-5 grade coal has the greatest capacity, while the capacity of the others is much lower and is approximately the same order. Considering the shortage of activated carbons and their high cost, other sorbents are being searched for. Currently, instead of activated carbon, the bioadsorbent C-verad is offered, which is not inferior to it in absorption capacity and is several times cheaper. Since C-verad immobilizes bacteria that process petroleum products into activated sludge, after a certain time there is no oil left in the spent adsorbent, so there are no problems with its disposal.
When using reagent flotation, the facilities are supplemented with a reagent facility (coagulant), similar to chemical water treatment. The coagulant is supplied before the flotation settler (in the energy sector, schemes using a coagulant have not been widely used due to the lack of significant effect in its use). Petroleum products and sediment released in installations are collected in special tanks, from where they are pumped for neutralization (incineration, burial).
The optimal type of structures, both from an economic point of view and taking into account the resulting quality of purification, are: sludge, flotation, mechanical filters and activated carbon filters, regenerated by steam - all devices are made of metal in a ground-based design. This scheme allows you to obtain a quality of purified water of no more than 1 mg/dm3, with an oil content of water supplied for treatment up to 100 mg/dm3.
· FROM washing of RVP and heating surfaces of boilers
Considering the presence of toxic substances in these wastewaters, it is necessary to ensure their neutralization and neutralization before discharge into the reservoir. The washing waters are sent to neutralizing tanks, and each neutralizing tank must contain the washing waters from washing one RVP and reagents for their treatment. The tanks provide for the precipitation of vanadium-containing sludge that meets the requirements of metallurgical plants.
At the first stage, neutralization is carried out with caustic soda to a pH value of 4.5...5, for the precipitation of vanadium oxides and the subsequent separation of vanadium-containing sludge - on filter presses of the FPAKM type. At the second stage, the clarified water of the first stage is treated with a lime solution to a pH value of 9.5...10 - to precipitate oxides of iron, nickel, copper, and calcium sulfate. The resulting sludge is sent to an unfiltered sludge dump, and the clarified water is reused for washing.
The average approximate size of the washing water flow for a large state district power plant is 10...15 t/h.
Chemical wastewater
One of the main disadvantages of these discharges is their sharply variable, “volley” flow rate and changing concentrations and composition of impurities during flushing. This leads to the need to have containers that, at a minimum, must be designed for the entire volume of discharged water, taking into account its threefold dilution.
The presence and concentrations of some impurities completely depend on the washing method (C1-, formaldehyde, hydrazine, etc.), while the concentrations of iron and foam formers are almost the same for all methods. For the convenience of selecting a method for treating wash waters, they can be divided into three groups based on the influence of the impurities they contain on the sanitary regime of water bodies:
1) inorganic substances, the concentration of which does not exceed their MPC values in water bodies; these are sulfates and chlorides of calcium, magnesium and sodium;
2) toxic substances, the content of which significantly exceeds their maximum permissible concentrations in water bodies; these are salts of iron, copper, zinc, fluorine-containing compounds, hydrazine;
3) organic substances, ammonium salts, nitrites, sulfides, which can be subject to bacterial or direct oxidation; The discharge of such substances must be calculated based on the BOD in the reservoir.
In practice, when neutralizing wash water, substances of the second group should be subjected to release, and substances of the third group should be oxidized to acceptable BOD.
Basically, the method of purifying washing and conservation waters depends on the type of fuel used and the adopted ash removal scheme. From this point of view, there are two options for treating such waters:
1) cleaning at thermal power plants operating on liquid and gas fuels, as well as at thermal power plants operating on solid fuel with an open-loop gas protection system;
2) cleaning at thermal power plants operating on solid fuel with a closed-loop gas control system. At gas-oil thermal power plants, water discharges from water washes containing coarse impurities must be directed into an open container to separate them, the volume of which is selected depending on the type of boiler and the volume of the washed circuits.
At gas-oil TPPs and TPPs with an open-loop GSU system, the wash water treatment scheme involves three stages:
1) collection of all spent solutions and part of the most contaminated wash water (pH< 6) в емкости-усреднители;
2) separation of toxic substances of the second group from solution
with sludge disposal in neutralizing tanks;
3) water purification from substances of the third group.
When neutralizing waste rinsing waters, the main tasks are the destruction of metal complexes with reagents formed during rinsing, the release of these metals into sediment and the destruction of organic compounds. Precipitation of heavy metal ions (Fe, Cu, Zn) is achieved by increasing the pH to 11.0 (lime solution) when solutions of hydrochloric, adipic, phthalic and dicarboxylic acids are used for washing. In the case of using a citrate solution at pH = 10, complete destruction of iron citrate complexes is observed. Complexes of copper and zinc with trilon are not destroyed over the entire pH range.
At thermal power plants with a closed gas treatment system, it is possible to discharge spent washing solutions directly to the ash dump if the pH of the clarified water of the ash dump is above 8.0. Otherwise, preliminary neutralization of the washing solutions is required. In any case, to prevent corrosion of the sump pumps, the pH value in the gas treatment system as a result of the discharge should not be lower than 7.0. Experimental data confirm the high adsorption capacity of ash towards impurities of the second and third groups.
Hydrazine, sodium nitrite and ammonia are present in large quantities in the waste water after equipment storage. A convenient way to decompose hydrazine is to treat the solution with bleach or liquid chlorine.
To carry out the process of cleaning discharged preservative solutions, the following scheme is used. The spent solution is collected in a tank, the capacity of which must be sufficient to receive its entire quantity at once. Tanks for preparing preservative solutions are used as such containers. If the cleaning process is organized in a neutralizer tank with a volume of about 20 m3, then reagents and steam are also sent to it. To speed up the process of cleaning and purging the solution with air with an ejection coefficient of at least 10, circulation is organized using a pump with a capacity of 80...150 m3/h and a pressure of up to 20 kgf/cm
with installation of a water-air ejector.
To decompose nitrite, sulfuric acid is introduced in an amount 10...15% greater than the stoichiometric one. It has been established that nitrite decomposes more intensively if the acid is supplied in two doses: first 50% of the calculated amount, and after 1 hour the rest. Air purging helps accelerate the decomposition of nitrite and hydrazine and removes ammonia. Increasing the temperature makes it possible to reduce the process of decomposition of impurities and the air consumption for purging gaseous components.
The disadvantage of acid neutralization is the formation of harmful nitrogen oxides, the disposal of which is not carried out with this scheme. A common drawback of the above-described purification processes for washing and preserving solutions is the high consumption of reagents, which significantly increases the salt content of the discharged water streams.
Over the past 15...20 years, an environmentally friendly method of pre-start and operational cleaning without the use of reagents, the so-called method of hot water-steam-oxygen cleaning and passivation of thermal power equipment, has been widely used. The method consists of treating surfaces with high-purity hot water (with electrical conductivity no more than 1 µS/cm) and steam at a certain temperature and speed and high oxygen concentrations (up to 2...3 g/dm3). As a result of this treatment, it is possible to remove deposits (up to 300 g/m2) and create a durable protective film on the metal, which has the same resistance to corrosion agents as stainless steel.
· Hydraulic ash removal systems
VTI has proposed a pilot industrial method for purifying GZU water from fluorine, vanadium, arsenic, and phenols, which consists of two stages. At the first stage, water is treated with lime and carbon dioxide from flue gases, which leads to the precipitation of calcium carbonate due to exceeding its solubility limits. At the same time, the fluorine content is partially reduced. The second stage consists of treating the resulting liquid with aluminum sulfate with a dosage of about 70 mg/dm3 in terms of the anhydrous product. This two-stage treatment makes it possible to reduce the fluorine content from 60 to 1.5 mg/dm3 and completely free it from vanadium, arsenic and phenols.
With the advent of closed gas storage systems, maintaining an optimal salt balance of the system has become very necessary and is carried out in various ways based on real conditions and economic considerations. Where possible, the system is purged into water bodies in compliance with the necessary conditions, as well as the purge water is evaporated using special devices. To remove deposits on pipelines and gas treatment plant equipment, water is treated with flue gases (cleaning the system of deposits). To prevent deposits, complexones (IOMS) are dosed, which in extremely small quantities prevent salt deposits.
Fuel supply path water
Polluted water is mainly subjected to settling, and clarified water is reused. The settled impurities and sludge are periodically removed by taking it to a coal stack.
Cleaning and reuse
surface runoff of thermal power plants
When choosing treatment schemes and using surface runoff, it is necessary to take into account the water balance of the power plant, the specifics of its operation (i.e., the required degree of wastewater treatment) and the economic feasibility of various options for treating and using these waters.
The occurrence of rain runoff necessitates the construction of a control tank. The scheme includes: a sand trap, a separation chamber, a drainage device, a control tank and a settling tank. If the technology for using surface runoff does not allow limiting the obtained depth of purification (sedimentation), it is necessary to provide additional filtration. The wastewater can be further purified using filters loaded with Kansk-Achinsk coal semi-coke (KAU) or anthracite.
Depending on the operating conditions of thermal power plants, the following main schemes for the use of surface runoff can be considered: in the recirculating cooling system, for feeding station water use systems (at chemical water treatment or in an evaporation plant), together with in-station oil-containing wastewater, for flushing ash and slag into the hydraulic ash removal system.
When using surface runoff to feed the recirculating cooling system, despite the increased mineralization of the runoff in certain periods, the carbonate alkalinity is relatively low, so feeding it into the recirculating system will not lead to a noticeable disruption of its water-chemical regime.
Surface runoff can be supplied to chemical water treatment with pre-treatment after settling; In water treatment plants without pre-treatment, additional filtration is required. If the power plant has facilities for treating oily wastewater, then surface runoff can be directed to them. In the presence of oil traps, the runoff is only accumulated; in their absence, it is sent to treatment facilities after settling. When surface water is supplied to the hydraulic ash removal system, only the accumulation of runoff is required. Purification and use of surface runoff in the power plant cycle makes it possible to reduce pollution of water bodies and water consumption of thermal power plants.